2011/109440 Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ligand-Specific Co-Receptor Function of CD44 for Receptor
The ligand-specific co-receptor function of CD44 for receptor tyrosine kinases Zur Erlangung des akademischen Grades eines DOKTORS DER NATURWISSENSCHAFTEN (Dr. rer. nat.) Fakultät für Chemie und Biowissenschaften Karlsruher Institut für Technologie (KIT) - Universitätsbereich genehmigte DISSERTATION von Christian Jung aus Karlsruhe Dekan: Prof. Dr. Martin Bastmeyer Referent: PD Dr. Véronique Orian-Rousseau Korreferent: Prof. Dr. Doris Wedlich Tag der mündlichen Prüfung: 18.4.2012 I Ich versichere, dass ich meine Arbeit selbständig angefertigt und keine anderen als die angegebenen Quellen und Hilfsmittel benutzt, sowie die wörtlich oder inhaltlich übernommenen Stellen als solche kenntlich gemacht und die Satzung der Universität Karlsruhe (TH) zur Sicherung guter wissenschaftlicher Praxis in der jeweils gültigen Fassung beachtet habe. Christian Jung, März 2012 II Zusammenfassung Listeria monocytogenes, ein gram-positives Bakterium, verursacht die Krankheit Listeriose. Eine Möglichkeit, wie L.monocytogenes Wirbeltierzellen infizieren kann, ist das Binden des Bakteriums an die Rezeportyrosinkinase (RTK) Met auf der Wirtszelle durch das bakterielle Protein InlB. Dieses Binden führt zur Aktivierung von Met und schließlich zur Aufnahme in die Zelle. Der erste Teil meine Doktorarbeit zeigt, dass die Infektion von nicht-phagozytotischen Zellen mittels InlB zusätzlich vom Ko-Rezeptor CD44v6 abhängig ist. Desweiteren kann diese bakterielle Infektion mit einem CD44v6-Peptid blockiert werden. Zusätzlich zu der Ko-Rezeptorfunktion von CD44v6 für InlB und Met, die ich gezeigt habe, wurde CD44v6 bereits als Ko-Rezeptor für die Induktion von Met und VEGFR- 2 durch ihre authentischen Liganden HGF and VEGF-A identifiziert. Im zweiten und Hauptteil meiner Doktorarbeit habe ich untersucht, ob diese Ko-Rezeptorfunktion von CD44v6 spezifisch von den Liganden, den Rezeptoren oder beiden bestimmt wird. -

Diagnostic Code Descriptions (ICD9)
INFECTIONS AND PARASITIC DISEASES INTESTINAL AND INFECTIOUS DISEASES (001 – 009.3) 001 CHOLERA 001.0 DUE TO VIBRIO CHOLERAE 001.1 DUE TO VIBRIO CHOLERAE EL TOR 001.9 UNSPECIFIED 002 TYPHOID AND PARATYPHOID FEVERS 002.0 TYPHOID FEVER 002.1 PARATYPHOID FEVER 'A' 002.2 PARATYPHOID FEVER 'B' 002.3 PARATYPHOID FEVER 'C' 002.9 PARATYPHOID FEVER, UNSPECIFIED 003 OTHER SALMONELLA INFECTIONS 003.0 SALMONELLA GASTROENTERITIS 003.1 SALMONELLA SEPTICAEMIA 003.2 LOCALIZED SALMONELLA INFECTIONS 003.8 OTHER 003.9 UNSPECIFIED 004 SHIGELLOSIS 004.0 SHIGELLA DYSENTERIAE 004.1 SHIGELLA FLEXNERI 004.2 SHIGELLA BOYDII 004.3 SHIGELLA SONNEI 004.8 OTHER 004.9 UNSPECIFIED 005 OTHER FOOD POISONING (BACTERIAL) 005.0 STAPHYLOCOCCAL FOOD POISONING 005.1 BOTULISM 005.2 FOOD POISONING DUE TO CLOSTRIDIUM PERFRINGENS (CL.WELCHII) 005.3 FOOD POISONING DUE TO OTHER CLOSTRIDIA 005.4 FOOD POISONING DUE TO VIBRIO PARAHAEMOLYTICUS 005.8 OTHER BACTERIAL FOOD POISONING 005.9 FOOD POISONING, UNSPECIFIED 006 AMOEBIASIS 006.0 ACUTE AMOEBIC DYSENTERY WITHOUT MENTION OF ABSCESS 006.1 CHRONIC INTESTINAL AMOEBIASIS WITHOUT MENTION OF ABSCESS 006.2 AMOEBIC NONDYSENTERIC COLITIS 006.3 AMOEBIC LIVER ABSCESS 006.4 AMOEBIC LUNG ABSCESS 006.5 AMOEBIC BRAIN ABSCESS 006.6 AMOEBIC SKIN ULCERATION 006.8 AMOEBIC INFECTION OF OTHER SITES 006.9 AMOEBIASIS, UNSPECIFIED 007 OTHER PROTOZOAL INTESTINAL DISEASES 007.0 BALANTIDIASIS 007.1 GIARDIASIS 007.2 COCCIDIOSIS 007.3 INTESTINAL TRICHOMONIASIS 007.8 OTHER PROTOZOAL INTESTINAL DISEASES 007.9 UNSPECIFIED 008 INTESTINAL INFECTIONS DUE TO OTHER ORGANISMS -

In Vivo Dual RNA-Seq Analysis Reveals the Basis for Differential Tissue Tropism of Clinical Isolates of Streptococcus Pneumoniae
In Vivo Dual RNA-Seq Analysis Reveals the Basis for Differential Tissue Tropism of Clinical Isolates of Streptococcus pneumoniae Vikrant Minhas,1,4 Rieza Aprianto,2,4 Lauren J. McAllister,1 Hui Wang,1 Shannon C. David,1 Kimberley T. McLean,1 Iain Comerford,3 Shaun R. McColl,3 James C. Paton,1,5,6,* Jan-Willem Veening,2,5 and Claudia Trappetti,1,5 Supplementary Information Supplementary Table 1. Pneumococcal differential gene expression in the lungs 6 h post-infection, 9-47-Ear vs 9-47M. Genes with fold change (FC) greater than 2 and p < 0.05 are shown. FC values highlighted in blue = upregulated in 9-47-Ear, while values highlighted in red = upregulated in 9- 47M. Locus tag in 9-47- Product padj FC Ear Sp947_chr_00844 Sialidase B 3.08E-10 313.9807 Sp947_chr_02077 hypothetical protein 4.46E-10 306.9412 Sp947_chr_00842 Sodium/glucose cotransporter 2.22E-09 243.4822 Sp947_chr_00841 N-acetylneuraminate lyase 4.53E-09 227.7963 scyllo-inositol 2-dehydrogenase Sp947_chr_00845 (NAD(+)) 4.36E-09 221.051 Sp947_chr_00848 hypothetical protein 1.19E-08 202.7867 V-type sodium ATPase catalytic subunit Sp947_chr_00853 A 1.29E-06 100.5411 Sp947_chr_00846 Beta-glucoside kinase 3.42E-06 98.18951 Sp947_chr_00855 V-type sodium ATPase subunit D 8.34E-06 85.94879 Sp947_chr_00851 V-type sodium ATPase subunit C 2.50E-05 72.46612 Sp947_chr_00843 hypothetical protein 2.17E-05 65.97758 Sp947_chr_00839 HTH-type transcriptional regulator RpiR 3.09E-05 61.28171 Sp947_chr_00854 V-type sodium ATPase subunit B 1.32E-06 50.86992 Sp947_chr_00120 hypothetical protein 3.00E-04 -

Investigation of Peptidyl-Prolyl Cis/Trans Isomerases in the Virulence of Staphylococcus
Investigation of Peptidyl-prolyl cis/trans isomerases in the virulence of Staphylococcus aureus A Dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Rebecca A. Keogh August 2020 © 2020 Rebecca A. Keogh. All Rights Reserved. 2 This Dissertation titled Investigation of Peptidyl-prolyl cis/trans isomerases in the virulence of Staphylococcus aureus by REBECCA A. KEOGH has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Ronan K. Carroll Assistant Professor of Biological Sciences Florenz Plassmann Dean, College of Arts and Sciences 3 ABSTRACT REBECCA A. KEOGH, Doctorate of Philosophy, August 2020, Biological Sciences Investigation of peptidyl-prolyl cis/trans isomerases in the virulence of Staphylococcus aureus Director of Dissertation: Ronan K. Carroll Staphylococcus aureus is a leading cause of both hospital and community- associated infections that can manifest in a wide range of diseases. These diseases range in severity from minor skin and soft tissue infections to life-threatening sepsis, endocarditis and meningitis. Of rising concern is the prevalence of antibiotic resistant S. aureus strains in the population, and the lack of new antibiotics being developed to treat them. A greater understanding of the ability of S. aureus to cause infection is crucial to better inform treatments and combat these antibiotic resistant superbugs. The ability of S. aureus to cause such diverse infections can be attributed to the arsenal of virulence factors produced by the bacterium that work to both evade the human immune system and assist in pathogenesis. -

Molecular Detection of Human Parasitic Pathogens
MOLECULAR DETECTION OF HUMAN PARASITIC PATHOGENS MOLECULAR DETECTION OF HUMAN PARASITIC PATHOGENS EDITED BY DONGYOU LIU Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2013 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Version Date: 20120608 International Standard Book Number-13: 978-1-4398-1243-3 (eBook - PDF) This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the validity of all materials or the consequences of their use. The authors and publishers have attempted to trace the copyright holders of all material reproduced in this publication and apologize to copyright holders if permission to publish in this form has not been obtained. If any copyright material has not been acknowledged please write and let us know so we may rectify in any future reprint. Except as permitted under U.S. Copyright Law, no part of this book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any information storage or retrieval system, without written permission from the publishers. For permission to photocopy or use material electronically from this work, please access www.copyright.com (http://www.copyright.com/) or contact the Copyright Clearance Center, Inc. -

Novel Therapeutic Interventions Towards Improved Management of Septic Arthritis Jian Wang1* and Liucai Wang2
Wang and Wang BMC Musculoskeletal Disorders (2021) 22:530 https://doi.org/10.1186/s12891-021-04383-6 REVIEW Open Access Novel therapeutic interventions towards improved management of septic arthritis Jian Wang1* and Liucai Wang2 Abstract Septic arthritis (SA) represents a medical emergency that needs immediate diagnosis and urgent treatment. Despite aggressive treatment and rapid diagnosis of the causative agent, the mortality and lifelong disability, associated with septic arthritis remain high as close to 11%. Moreover, with the rise in drug resistance, the rates of failure of conventional antibiotic therapy have also increased. Among the etiological agents frequently isolated from cases of septic arthritis, Staphylococcus aureus emerges as a dominating pathogen, and to worsen, the rise in methicillin- resistant S. aureus (MRSA) isolates in bone and joint infections is worrisome. MRSA associated cases of septic arthritis exhibit higher mortality, longer hospital stay, and higher treatment failure with poorer clinical outcomes as compared to cases caused by the sensitive strain i.e methicillin-sensitive S. aureus (MSSA). In addition to this, equal or even greater damage is imposed by the exacerbated immune response mounted by the patient’s body in a futile attempt to eradicate the bacteria. The antibiotic therapy may not be sufficient enough to control the progression of damage to the joint involved thus, adding to higher mortality and disability rates despite the prompt and timely start of treatment. This situation implies that efforts and focus towards studying/ understanding new strategies for improved management of sepsis arthritis is prudent and worth exploring. The review article aims to give a complete insight into the new therapeutic approaches studied by workers lately in this field. -
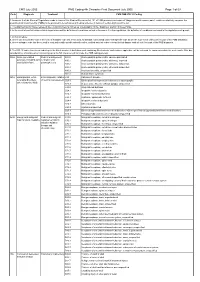
CMS PMB ICD-10 Coding
CMS July 2005 PMB Coding 4th Character Final Dcoument July 2005 Page 1 of 69 Code Diagnosis Treatment CMS PMB ICD-10 Coding 1. Annexure A of the General Regulations made in terms of the Medical Schemes Act, 131 of 1998 provides a schedule of “diagnosis and treatment pairs”, which cumulatively comprise the prescribed minimum benefits (PMBs) to be provided to beneficiaries of medical schemes in terms of section 29(1)(o) of the Act. 2. The attached ICD10 codes represent the Council for Medical Schemes’ interpretation of the “diagnosis” portion of these PMBs. 3. In the event of conflict between this interpretation and the definition of conditions set out in Annexure A to the regulations, the definition of conditions contained in the regulations will prevail. 4. In this schedule: a. where only the primary code in the form of a dagger code has been used, all asterisk codes listed under that specific code as per the ICD-10 set of books form part of the PMB diagnosis; b. where a dagger code has been used in conjunction with specific asterisk codes, omitted asterisk codes relevant to that dagger code do not form part of the PMB diagnosis. 5. The ICD-10 codes have been coded up to the 4th character. A draft document containing 5th character codes where applicable, will be released for comments within the next month. After due consideration, a final document containing up to the 5th character will conclude the PMB coding process. 906A Acute generalised Medical management; A80.0 Acute paralytic poliomyelitis, vaccine-associated paralysis, including -

Signal Transduction, Quorum-Sensing, and Extracellular Protease Activity in Enterococcus Faecalis Biofilm Formation
Signal Transduction, Quorum-Sensing, and Extracellular Protease Activity in Enterococcus faecalis Biofilm Formation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Carniol, K., and M. S. Gilmore. 2004. Signal Transduction, Quorum- Sensing, and Extracellular Protease Activity in Enterococcus Faecalis Biofilm Formation. Journal of Bacteriology 186, no. 24: 8161–8163. doi:10.1128/jb.186.24.8161-8163.2004. Published Version doi:10.1128/JB.186.24.8161-8163.2004 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:33867369 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA JOURNAL OF BACTERIOLOGY, Dec. 2004, p. 8161–8163 Vol. 186, No. 24 0021-9193/04/$08.00ϩ0 DOI: 10.1128/JB.186.24.8161–8163.2004 Copyright © 2004, American Society for Microbiology. All Rights Reserved. GUEST COMMENTARY Signal Transduction, Quorum-Sensing, and Extracellular Protease Activity in Enterococcus faecalis Biofilm Formation Karen Carniol1,2 and Michael S. Gilmore1,2* Department of Ophthalmology, Harvard Medical School,1 and The Schepens Eye Research Institute,2 Boston, Massachusetts Biofilms are surface-attached communities of bacteria, en- sponse regulator proteins (10). Only one of the mutants gen- cased in an extracellular matrix of secreted proteins, carbohy- erated, fsrA, impaired the ability of E. faecalis strain V583A to drates, and/or DNA, that assume phenotypes distinct from form biofilms in vitro. -

Host and Bacterial Determinants of Staphylococcus Aureus Nasal Colonization in Humans
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2014 Host and Bacterial Determinants of Staphylococcus aureus Nasal Colonization in Humans Gowrishankar Muthukrishnan University of Central Florida Part of the Medical Sciences Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Muthukrishnan, Gowrishankar, "Host and Bacterial Determinants of Staphylococcus aureus Nasal Colonization in Humans" (2014). Electronic Theses and Dissertations, 2004-2019. 1289. https://stars.library.ucf.edu/etd/1289 HOST AND BACTERIAL DETERMINANTS OF STAPHYLOCOCCUS AUREUS NASAL COLONIZATION IN HUMANS by GOWRISHANKAR MUTHUKRISHNAN M.E. Birla Institute of Technology and Science, Pilani, India, 2007 M.S. University of Central Florida, United States of America, 2010 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Burnett School of Biomedical Sciences in the College of Medicine at the University of Central Florida Orlando, Florida Summer Term 2014 Major Professor: Alexander M. Cole © 2014 Gowrishankar Muthukrishnan ii ABSTRACT Staphylococcus aureus (SA), an opportunistic pathogen colonizing the anterior nares in approximately 30% of the human population, causes severe hospital-associated and community-acquired infections. SA nasal carriage plays a critical role in the pathogenesis of staphylococcal infections and SA eradication from the nares has proven to be effective in reducing endogenous infections. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110747 A1 Ramseier Et Al
US 200601 10747A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110747 A1 Ramseier et al. (43) Pub. Date: May 25, 2006 (54) PROCESS FOR IMPROVED PROTEIN (60) Provisional application No. 60/591489, filed on Jul. EXPRESSION BY STRAIN ENGINEERING 26, 2004. (75) Inventors: Thomas M. Ramseier, Poway, CA Publication Classification (US); Hongfan Jin, San Diego, CA (51) Int. Cl. (US); Charles H. Squires, Poway, CA CI2O I/68 (2006.01) (US) GOIN 33/53 (2006.01) CI2N 15/74 (2006.01) Correspondence Address: (52) U.S. Cl. ................................ 435/6: 435/7.1; 435/471 KING & SPALDING LLP 118O PEACHTREE STREET (57) ABSTRACT ATLANTA, GA 30309 (US) This invention is a process for improving the production levels of recombinant proteins or peptides or improving the (73) Assignee: Dow Global Technologies Inc., Midland, level of active recombinant proteins or peptides expressed in MI (US) host cells. The invention is a process of comparing two genetic profiles of a cell that expresses a recombinant (21) Appl. No.: 11/189,375 protein and modifying the cell to change the expression of a gene product that is upregulated in response to the recom (22) Filed: Jul. 26, 2005 binant protein expression. The process can improve protein production or can improve protein quality, for example, by Related U.S. Application Data increasing solubility of a recombinant protein. Patent Application Publication May 25, 2006 Sheet 1 of 15 US 2006/0110747 A1 Figure 1 09 010909070£020\,0 10°0 Patent Application Publication May 25, 2006 Sheet 2 of 15 US 2006/0110747 A1 Figure 2 Ester sers Custer || || || || || HH-I-H 1 H4 s a cisiers TT closers | | | | | | Ya S T RXFO 1961. -

A Bacterial Goldilocks Mechanism
INSIGHT TWO-COMPONENT SIGNALING PATHWAYS A bacterial Goldilocks mechanism Bacillus subtilis can measure the activity of the enzymes that remodel the cell wall to ensure that the levels of activity are ‘just right’. IRENE M KIM AND HENDRIK SZURMANT detector and a transcription factor that commu- Related research article Dobihal GS, Bru- nicate with one another through the transfer of a net YR, Flores-Kim J, Rudner DZ. 2019. phosphoryl group. Homeostatic control of cell wall hydrolysis An important TCS in the soil bacterium Bacil- by the WalRK two-component signaling lus subtilis and other related bacteria is the pathway in Bacillus subtilis. eLife 8:e52088. WalRK system, which is essential for viability DOI: 10.7554/eLife.52088 (Szurmant, 2012). The system, which is com- prised of the signal-detecting protein WalK and the transcription factor WalR, gets its name from its role in maintaining the cell wall (Dubrac et al., 2007). Together these two com- hen mollusks get bigger, their shells ponents regulate the expression of several auto- grow with them to accommodate lysin genes, including those for the enzymes W the changing shape and size of the LytE and CwlO, which are required for cell elon- organism being housed. Something similar also gation (Salzberg et al., 2013). Now, in eLife, happens in bacteria. The cell wall of most bacte- David Rudner and colleagues at Harvard Medical ria consists of a single macromolecule called School – including Genevieve Dobihal and Yan- peptidoglycan that surrounds the cell and is nick Brunet as joint first authors, along with made up of modified sugars that are crosslinked Josue´ Flores-Kim – report on how the WalRK through peptide side chains. -

The Characterization of a Putative Protease Expressed by Sneathia Amnii
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2015 The Characterization of a Putative Protease Expressed by Sneathia amnii Rana Mehr Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Medicine and Health Sciences Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/3931 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. CHARACTERIZATION OF A PUTATIVE PROTEASE EXPRESSED BY SNEATHIA AMNII A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science at Virginia Commonwealth University by RANA MEHR B.S., Virginia Commonwealth University 2011 Director: Kimberly Jefferson, Ph.D. Associate Professor, Department of Microbiology and Immunology Virginia Commonwealth University Richmond, Virginia Virginia Commonwealth University Richmond, Virginia July, 2015 Acknowledgements I would first like to express my deepest gratitude to my mentor Dr. Kimberly Jefferson. Her continuous mentorship, trust, and support in academic, scientific, and personal experiences have empowered me to successfully complete my graduate career both academically and scientifically. She has aided my development as an independent scientist which would have not been possible without guidance. I would also like to thank the members of my graduate advisory committee: Dr. Dennis Ohman and Dr. Darrell Peterson. Their advice and direction have allowed me to better understand my project and their invaluable knowledge has made me a better scientist.