Downloaded From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Maria Khayutina • [email protected] the Tombs
Maria Khayutina [email protected] The Tombs of Peng State and Related Questions Paper for the Chicago Bronze Workshop, November 3-7, 2010 (, 1.1.) () The discovery of the Western Zhou period’s Peng State in Heng River Valley in the south of Shanxi Province represents one of the most fascinating archaeological events of the last decade. Ruled by a lineage of Kui (Gui ) surname, Peng, supposedly, was founded by descendants of a group that, to a certain degree, retained autonomy from the Huaxia cultural and political community, dominated by lineages of Zi , Ji and Jiang surnames. Considering Peng’s location right to the south of one of the major Ji states, Jin , and quite close to the eastern residence of Zhou kings, Chengzhou , its case can be very instructive with regard to the construction of the geo-political and cultural space in Early China during the Western Zhou period. Although the publication of the full excavations’ report may take years, some preliminary observations can be made already now based on simplified archaeological reports about the tombs of Peng ruler Cheng and his spouse née Ji of Bi . In the present paper, I briefly introduce the tombs inventory and the inscriptions on the bronzes, and then proceed to discuss the following questions: - How the tombs M1 and M2 at Hengbei can be dated? - What does the equipment of the Hengbei tombs suggest about the cultural roots of Peng? - What can be observed about Peng’s relations to the Gui people and to other Kui/Gui- surnamed lineages? 1. General Information The cemetery of Peng state has been discovered near Hengbei village (Hengshui town, Jiang County, Shanxi ). -

Chen Gui and Other Works Attributed to Empress Wu Zetian
chen gui denis twitchett Chen gui and Other Works Attributed to Empress Wu Zetian ome quarter-century ago, studies by Antonino Forte and Richard S Guisso greatly advanced our understanding of the ways in which the empress Wu Zetian ࣳঞ֚ made deliberate and sophisticated use of Buddhist materials both before and after declaring herself ruler of a new Zhou ࡌʳdynasty in 690, in particular the text of Dayun jing Օႆᆖ in establishing her claim to be a legitimate sovereign.1 However, little attention has ever been given to the numerous political writings that had earlier been compiled in her name. These show that for some years before the demise of her husband emperor Gaozong in 683, she had been at considerable pains to establish her credentials as a potential ruler in more conventional terms, and had commissioned the writing of a large series of political writings designed to provide the ideologi- cal basis for both a new style of “Confucian” imperial rule and a new type of minister. All save two of these works were long ago lost in China, where none of her writings seems to have survived the Song, and most may not have survived the Tang. We are fortunate enough to possess that titled complete with its commentary, and also a fragmentary Chen gui copy of the work on music commissioned in her name, Yue shu yaolu ᑗ ᙕ,2 only thanks to their preservation in Japan. They had been ac- quired by an embassy to China, almost certainly that of 702–704, led టԳ (see the concluding section of thisضby Awata no ason Mahito ொ article) to the court of empress Wu, who was at that time sovereign of 1 See Antonino Forte, Political Propaganda and Ideology in China at the End of the Seventh Century (Naples: Istituto Universitario Orientale,1976); R. -

A Dictionary of Chinese Characters: Accessed by Phonetics
A dictionary of Chinese characters ‘The whole thrust of the work is that it is more helpful to learners of Chinese characters to see them in terms of sound, than in visual terms. It is a radical, provocative and constructive idea.’ Dr Valerie Pellatt, University of Newcastle. By arranging frequently used characters under the phonetic element they have in common, rather than only under their radical, the Dictionary encourages the student to link characters according to their phonetic. The system of cross refer- encing then allows the student to find easily all the characters in the Dictionary which have the same phonetic element, thus helping to fix in the memory the link between a character and its sound and meaning. More controversially, the book aims to alleviate the confusion that similar looking characters can cause by printing them alongside each other. All characters are given in both their traditional and simplified forms. Appendix A clarifies the choice of characters listed while Appendix B provides a list of the radicals with detailed comments on usage. The Dictionary has a full pinyin and radical index. This innovative resource will be an excellent study-aid for students with a basic grasp of Chinese, whether they are studying with a teacher or learning on their own. Dr Stewart Paton was Head of the Department of Languages at Heriot-Watt University, Edinburgh, from 1976 to 1981. A dictionary of Chinese characters Accessed by phonetics Stewart Paton First published 2008 by Routledge 2 Park Square, Milton Park, Abingdon, OX14 4RN Simultaneously published in the USA and Canada by Routledge 270 Madison Ave, New York, NY 10016 Routledge is an imprint of the Taylor & Francis Group, an informa business This edition published in the Taylor & Francis e-Library, 2008. -

Ideophones in Middle Chinese
KU LEUVEN FACULTY OF ARTS BLIJDE INKOMSTSTRAAT 21 BOX 3301 3000 LEUVEN, BELGIË ! Ideophones in Middle Chinese: A Typological Study of a Tang Dynasty Poetic Corpus Thomas'Van'Hoey' ' Presented(in(fulfilment(of(the(requirements(for(the(degree(of(( Master(of(Arts(in(Linguistics( ( Supervisor:(prof.(dr.(Jean=Christophe(Verstraete((promotor)( ( ( Academic(year(2014=2015 149(431(characters Abstract (English) Ideophones in Middle Chinese: A Typological Study of a Tang Dynasty Poetic Corpus Thomas Van Hoey This M.A. thesis investigates ideophones in Tang dynasty (618-907 AD) Middle Chinese (Sinitic, Sino- Tibetan) from a typological perspective. Ideophones are defined as a set of words that are phonologically and morphologically marked and depict some form of sensory image (Dingemanse 2011b). Middle Chinese has a large body of ideophones, whose domains range from the depiction of sound, movement, visual and other external senses to the depiction of internal senses (cf. Dingemanse 2012a). There is some work on modern variants of Sinitic languages (cf. Mok 2001; Bodomo 2006; de Sousa 2008; de Sousa 2011; Meng 2012; Wu 2014), but so far, there is no encompassing study of ideophones of a stage in the historical development of Sinitic languages. The purpose of this study is to develop a descriptive model for ideophones in Middle Chinese, which is compatible with what we know about them cross-linguistically. The main research question of this study is “what are the phonological, morphological, semantic and syntactic features of ideophones in Middle Chinese?” This question is studied in terms of three parameters, viz. the parameters of form, of meaning and of use. -
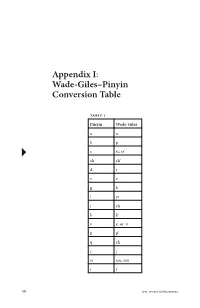
Appendix I: Wade-Giles–Pinyin Conversion Table
Appendix I: Wade-Giles–Pinyin Conversion Table Table 1 Pinyin Wade-Giles aa bp c ts’, tz’ ch ch’ dt ee gk iyi jch kk’ oe or o pp’ qch rj si ssu, szu tt’ 58 DOI: 10.1057/9781137303394 Appendix I: Wade-Giles–Pinyin Conversion Table 59 xhs yi i yu u, yu you yu z ts, tz zh ch zi tzu -i (zhi) -ih (chih) -ie (lie) -ieh (lieh) -r (er) rh (erh) Examples jiang chiang zhiang ch’iang zi tzu zhi chih cai tsai Zhu Xi Chu Hsi Xunzi Hsün Tzu qing ch’ing xue hsüeh DOI: 10.1057/9781137303394 60 Appendix I: Wade-Giles–Pinyin Conversion Table Table 2 Wade–Giles Pinyin aa ch’ ch ch j ch q ch zh ee e or oo ff hh hs x iyi -ieh (lieh) -ie (lie) -ih (chih) -i (zhi) jr kg k’ k pb p’ p rh (erh) -r (er) ssu, szu si td t’ t ts’, tz’ c ts, tz z tzu zi u, yu u yu you DOI: 10.1057/9781137303394 Appendix I: Wade-Giles–Pinyin Conversion Table 61 Examples chiang jiang ch’iang zhiang ch‘ing qing chih zhi Chu Hsi Zhu Xi hsüeh xue Hsün Tzu Xunzi tsai cai tzu zi DOI: 10.1057/9781137303394 Appendix II: Concordance of Key Philosophical Terms ⠅ai (To love) 1.5, 1.6, 3.17, 12.10, 12.22, 14.7, 17.4, 17.21 (9) 䘧 dao (Way, Path, Road, The Way, To tread a path, To speak, Doctrines, etc.) 1.2, 1.5, 1.11, 1.12, 1.14, 1.15, 2.3, 3.16, 3.24, 4.5, 4.8, 4.9, 4.15, 4.20, 5.2, 5.7, 5.13, 5.16, 5.21, 6.12, 6.17, 6.24, 7.6, 8.4, 8.7, 8.13, 9.12, 9.27, 9.30, 11.20, 11.24, 12.19, 12.23, 13.25, 14.1, 14.3, 14.19, 14.28, 14.36, 15.7, 15.25, 15.29, 15.32, 15.40, 15.42, 16.2, 16.5, 16.11, 17.4, 17.14, 18.2, 18.5, 18.7, 19.2, 19.4, 19.7, 19.12, 19.19, 19.22, 19.25. -

CHINESE ARTISTS Pinyin-Wade-Giles Concordance Wade-Giles Romanization of Artist's Name Dates R Pinyin Romanization of Artist's
CHINESE ARTISTS Pinyin-Wade-Giles Concordance Wade-Giles Romanization of Artist's name ❍ Dates ❍ Pinyin Romanization of Artist's name Artists are listed alphabetically by Wade-Giles. This list is not comprehensive; it reflects the catalogue of visual resource materials offered by AAPD. Searches are possible in either form of Romanization. To search for a specific artist, use the find mode (under Edit) from the pull-down menu. Lady Ai-lien ❍ (late 19th c.) ❍ Lady Ailian Cha Shih-piao ❍ (1615-1698) ❍ Zha Shibiao Chai Ta-K'un ❍ (d.1804) ❍ Zhai Dakun Chan Ching-feng ❍ (1520-1602) ❍ Zhan Jingfeng Chang Feng ❍ (active ca.1636-1662) ❍ Zhang Feng Chang Feng-i ❍ (1527-1613) ❍ Zhang Fengyi Chang Fu ❍ (1546-1631) ❍ Zhang Fu Chang Jui-t'u ❍ (1570-1641) ❍ Zhang Ruitu Chang Jo-ai ❍ (1713-1746) ❍ Zhang Ruoai Chang Jo-ch'eng ❍ (1722-1770) ❍ Zhang Ruocheng Chang Ning ❍ (1427-ca.1495) ❍ Zhang Ning Chang P'ei-tun ❍ (1772-1842) ❍ Zhang Peitun Chang Pi ❍ (1425-1487) ❍ Zhang Bi Chang Ta-ch'ien [Chang Dai-chien] ❍ (1899-1983) ❍ Zhang Daqian Chang Tao-wu ❍ (active late 18th c.) ❍ Zhang Daowu Chang Wu ❍ (active ca.1360) ❍ Zhang Wu Chang Yü [Chang T'ien-yu] ❍ (1283-1350, Yüan Dynasty) ❍ Zhang Yu [Zhang Tianyu] Chang Yü ❍ (1333-1385, Yüan Dynasty) ❍ Zhang Yu Chang Yu ❍ (active 15th c., Ming Dynasty) ❍ Zhang You Chang Yü-ts'ai ❍ (died 1316) ❍ Zhang Yucai Chao Chung ❍ (active 2nd half 14th c.) ❍ Zhao Zhong Chao Kuang-fu ❍ (active ca. 960-975) ❍ Zhao Guangfu Chao Ch'i ❍ (active ca.1488-1505) ❍ Zhao Qi Chao Lin ❍ (14th century) ❍ Zhao Lin Chao Ling-jang [Chao Ta-nien] ❍ (active ca. -

Researching Your Asian Roots for Chinese-Americans
Journal of East Asian Libraries Volume 2003 Number 129 Article 6 2-1-2003 Researching Your Asian Roots for Chinese-Americans Sheau-yueh J. Chao Follow this and additional works at: https://scholarsarchive.byu.edu/jeal BYU ScholarsArchive Citation Chao, Sheau-yueh J. (2003) "Researching Your Asian Roots for Chinese-Americans," Journal of East Asian Libraries: Vol. 2003 : No. 129 , Article 6. Available at: https://scholarsarchive.byu.edu/jeal/vol2003/iss129/6 This Article is brought to you for free and open access by the Journals at BYU ScholarsArchive. It has been accepted for inclusion in Journal of East Asian Libraries by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. researching YOUR ASIAN ROOTS CHINESE AMERICANS sheau yueh J chao baruch college city university new york introduction situated busiest mid town manhattan baruch senior colleges within city university new york consists twenty campuses spread five boroughs new york city intensive business curriculum attracts students country especially those asian pacific ethnic background encompasses nearly 45 entire student population baruch librarian baruch college city university new york I1 encountered various types questions serving reference desk generally these questions solved without major difficulties however beginning seven years ago unique question referred me repeatedly each new semester began how I1 find reference book trace my chinese name resource english help me find origin my chinese surname -

NCCAOM Reference of Common Chinese Herbal Formulas
NCCAOM test preparation reference NCCAOM Reference of Common Chinese Herbal Formulas � Ba Zhen Tang (Eight-Treasure Decoction) � Ba Zheng San (Eight-Herb Powder for Rectification) � Bai He Gu Jin Tang (Lily Bulb Decoction to Preserve the Metal) � Bai Hu Tang (White Tiger Decoction) � Bai Tou Weng Tang (Pulsatilla Decoction) � Ban Xia Bai Zhu Tian Ma Tang (Pinellia, Atractylodis Macrocephalae, and Gastrodia Decoction) � Ban Xia Hou Po Tang (Pinellia and Magnolia Bark Decoction) � Ban Xia Xie Xin Tang (Pinellia Decoction to Drain the Epigastrium) � Bao He Wan (Preserve Harmony Pill) � Bu Yang Huan Wu Tang (Tonify the Yang to Restore Five (Tenths) Decoction) � Bu Zhong Yi Qi Tang (Tonify the Middle and Augment the Qi Decoction) � Cang Er Zi San (Xanthium Powder) � Chai Ge Jie Ji Tang (Bupleurum and Kudzu Decoction) � Chai Hu Shu Gan San (Bupleurum Powder to Spread the Liver) � Chuan Xiong Cha Tiao San (Ligusticum Chuanxiong Powder to be Taken with Green Tea) � Da Bu Yin Wan (Great Tonify the Yin Pill) � Da Chai Hu Tang (Major Bupleurum Decoction) � Da Cheng Qi Tang (Major Order the Qi Decoction) � Da Jian Zhong Tang (Major Construct the Middle Decoction) � Dang Gui Bu Xue Tang (Tangkuei Decoction to Tonify the Blood) � Dang Gui Liu Huang Tang (Tangkuei and Six-Yellow Decoction) � Dao Chi San (Guide Out the Red Powder) � Ding Chuan Tang (Arrest Wheezing Decoction) � Du Huo Ji Sheng Tang (Angelica Pubescens and Sangjisheng Decoction) � Du Qi Wan (Capital Qi Pill) � Er Chen Tang (Two-Cured Decoction) � Er Miao San (Two-Marvel Powder) � Er Xian -

Di Zi Gui � (Students' Rules)
1 Di Zi Gui (Students' Rules) By Li Yue Xiu Of the Qing Dynasty, during the Reign of the Emperor Kangxi (1661-1722) With Mandarin Pinyin and Cantonese Pronunciation Translation and Transliteration by Feng Xin-ming 1989,1993, 2000, 2004, 2006, Jan., May & July 2008, 2009 (Complicated Chinese Script Version) To Simplified Chinese Script 到简体页: http://www.tsoidug.org/dizigui/dizigui_web_simp.pdf Complicated Chinese Script : http://www.tsoidug.org/dizigui/dizigui_web.pdf Return to Di Zi Gui Page : http://www.tsoidug.org/dizigui_comp.php 2 Translator's Note Being one of the two or three most popular primers with which youngsters were taught how to be gentlemen during the last two centuries of imperial China, Di Zi Gui is a treasure trove of traditional Chinese thought, norms and mores. Upon first reading Di Zi Gui in the 1980's, I exclaimed, "I've found it! I have finally found what my children need!" So I taught them Di Zi Gui. Since they were born and growing up in North America, I had to translate and transliterate the Chinese text. From that early work and subsequent refinements came this piece on Di Zi Gui. Looking back, I think learning Di Zi Gui as children has helped them a lot in their behavior, their ability to get along with others, and their self-esteem. Di Zi Gui has helped give them a sense of pride and identity, a quiet confidence that what they are doing is right, a self-generated drive to always do their best, and an inner strength to overcome adversity. -

Di Zi Gui with English, Pinyin & Cantonese Pronunciation
1 Di Zi Gui (Students' Rules) 规 By Li Yue Xiu Of the Qing Dynasty, during the Reign of the Emperor Kangxi (1661-1722) With Mandarin Pinyin and Cantonese Pronunciation 间 Translation and Transliteration by Feng Xin-ming 1989, 1993, 2000, 2004, 2006, Jan., May, & July 2008, 2009 (Simplified Chinese Script Version) With Mandarin Pinyin and Cantonese Pronunciation 冯欣明英语翻译 To Complicated Chinese Script : http://www.tsoidug.org/dizigui/dizigui_web.pdf 附拼音及粤音 Simplified Chinese Script 简体页: http://www.tsoidug.org/dizigui/dizigui_web_simp.pdf (简体版) Return to Di Zi Gui Page : http://www.tsoidug.org/dizigui.php 2 Translator's Note Being one of the two or three most popular primers with which youngsters were taught how to be gentlemen during the last two centuries of imperial China, Di Zi Gui is a treasure trove of traditional Chinese thought, norms and mores. Upon first reading Di Zi Gui in the 1980's, I exclaimed, "I've found it! I have finally found what my children need!" So I taught them Di Zi Gui. Since they were born and growing up in North America, I had to translate and transliterate the Chinese text. From that early work and subsequent refinements came this piece on Di Zi Gui. Looking back, I think learning Di Zi Gui as children has helped them a lot in their behavior, their ability to get along with others, and their self-esteem. Di Zi Gui has helped give them a sense of pride and identity, a quiet confidence that what they are doing is right, a self-generated drive to always do their best, and an inner strength to overcome adversity. -

Spaced Learning Enhances Episodic Memory by Increasing Neural Pattern Similarity Across Repetitions
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Behavioral/Cognitive Spaced learning enhances episodic memory by increasing neural pattern similarity across repetitions Kanyin Fenga, Xiao Zhaoa, Jing Liua, Ying Caia, Zhifang Yea, Chuansheng Chenb and Gui Xuea aState Key Laboratory of Cognitive Neuroscience and Learning & IDG/McGovern Institute for Brain Research, Beijing Normal University, Beijing, 100875, China bDepartment of Psychological Science, University of California, Irvine, CA, 92697, USA https://doi.org/10.1523/JNEUROSCI.2741-18.2019 Received: 22 October 2018 Revised: 19 April 2019 Accepted: 23 April 2019 Published: 29 April 2019 Author contributions: K.F., X.Z.nd G.X. designed research; K.F., J.L.nd G.X. analyzed data; K.F., C.C.nd G.X. wrote the paper; X.Z., J.L., Y.C.nd Z.Y. performed research. Conflict of Interest: The authors declare no competing financial interests. This work was sponsored by the National Science Foundation of China (31730038, 31861143040), the 111 Project (B07008), the NSFC and the German Research Foundation (DFG) joint project NSFC 61621136008/ DFG TRR-169nd the Guangdong Pearl River Talents Plan Innovative and Entrepreneurial Team grant #2016ZT06S220. Correspondence author: Gui Xue, State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing, 100875, China. Tel. & Fax. 8610-58801859. Email: [email protected] Cite as: J. Neurosci 2019; 10.1523/JNEUROSCI.2741-18.2019 Alerts: Sign up at www.jneurosci.org/alerts to receive customized email alerts when the fully formatted version of this article is published. -

42 Wade–Giles Romanization System
42 Wade–Giles ROMANIZATION SYSTEM Karen Steffen Chung N ATIONAL TAIWAN UNIVERSITY The Wade–Giles Romanization system for standard Mandarin Chinese held a distinguished place of honor in Sinology and popular usage from the late nineteenth century until the 1970s, when it began losing ground to Hanyu Pinyin. But that is not to say that the Wade– Giles system was not, and is not still, without its problems, and consequently, its sometimes highly vocal detractors. Historical absence of a phonetic alphabet, fanqie and tone marking It is surprising that the Chinese did not develop their own phonetic alphabet before the arrival of Western missionaries in China starting in the sixteenth century. The closest they came was the use of the fǎnqiè system, under which two relatively well-known characters, plus the word fǎn 反 or later mostly qiè 切, were given after a lexical item. The reader needed to take the initial of the first and splice it onto the final rhyme and tone of the second, to derive the pronunciation of the item being looked up. A typical entry is dōng déhóng qiè 東 德紅切, i.e. dé plus hóng in the qiè 切 system make dōng. (The second tone had not yet separated from the first at this time, thus the difference in tones.) One big advantage of the system is that the fǎnqiè characters were already familiar to any literate Chinese, so there was no need to learn a new set of symbols. The disadvantage is that there is no way to know with certainty the actual phonetic realizations of the syllables at the time .