MIAMI UNIVERSITY
The Graduate School
Certificate for Approving the Dissertation
We hereby approve the Dissertation of
Matthew F. Rouhier
Candidate for the Degree:
Doctor of Philosophy
___________________________________________________________
Director
(Dr. Ann Hagerman)
___________________________________________________________
Reader
(Dr.Chris Makaroff)
___________________________________________________________
Reader
(Dr.Gary Lorigan)
__________________________________________________________
Reader
(Dr. Richard Taylor)
_________________________________________________________
Graduate School Representative
(Dr. Paul James)
!
ABSTRACT
CHARACTERIZATION OF YDR036C FROM Saccharomyces cerevisiae
by Matthew F. Rouhier
Beta-hydroxyisobutyryl-CoA (HIBYL-CoA) hydrolases are found ubiquitously in eukaryotes where they function in the catabolism of valine. A homologous enzyme (YDR036C) is also present in yeast where valine catabolism is distinctly different and does not require a HIBYL-CoA hydrolase. Like the other eukaryotic hydrolases, the yeast hydrolase is a member of the crotonase super-family which catalyzes various reactions using CoA thioester substrates. Crotonases typically function in fatty acid degradation which is peroxisomal in yeast while YDR036C is found strictly in mitochondria. Like other HIBYL-CoA hydrolases the yeast enzyme demonstrated activity toward beta-hydroxyacyl-CoAs, but unlike the other HIBYL-CoA hydrolases the yeast activity is greatest with beta-hydroxypropionyl-CoA (3-HP-CoA). Further characterization of the active site has determined that the preference of the hydrolases for 3-HP- CoA or HIBYL-CoA is dependent upon the residue at position 177. The glutamate at position 121 is responsible for the coordination with the beta-hydroxyl group and replacement with valine renders the enzyme non-specific for a hydroxyl group. Another unique feature to yeast hydrolases is the presence of a C-terminal tail of 80 amino acids, which when removed renders the enzyme inactive, suggesting that it may play a role in substrate binding. Serial truncation of the enzyme demonstrated that the tail residues between 472 and 500 are critical for the proper hydrolase activity. Similarly the enzyme can be inactivated by placing a phosphate ester-mimic in place of serine 428. Although the active site seems optimized for 3-HP-CoA this compound is not a known metabolite of yeast. This suggests that YDR036C may be serving a unique function in yeast. High-throughput studies have identified a mild phenotype in fluid-phase endoyctosis in ydr036c mutants. The same phenotype is observed in knockouts of ergosterol synthesis enzymes. Ergosterol quantification in the ydr036c knockout showed reduced total cellular ergosterol when compared to wild-type. Ergosterol was subsequently docked to a model of the enzyme and shown to bind at the active site pocket. Since the storage mechanism for yeast sterols is sterol esters, this study proposes that YDR036C is not a HIBYL-CoA hydrolase, but instead may function as a mitochondrial sterol esterase.
!
CHARACTERIZATION OF YDR036C
FROM Saccharomyces cerevisiae
A DISSERTATION
Submitted to the Faculty of Miami University in partial Fulfillment of the requirements for a degree of
Doctor of Philosophy
Department of Chemistry and Biochemistry
by
Matthew F. Rouhier Miami University
Oxford, Ohio
2011
!
Table of Contents
Chapter 1:
1.1
1.1.1
1.2
1.2.1
Introduction .............................................................................................................1
Crotonase.........................................................................................................................1
Crotonase.................................................................................................................1
!-hydroxyisobutyryl-CoA hydrolase...............................................................................1
HIBYL-CoA hydrolase homology ..........................................................................1 HIBYL-CoA hydrolase and valine metabolism ......................................................2 HIBYL-CoA hydrolase structure ............................................................................2 HIBYL-CoA hydrolase mechanism ........................................................................2 HIBYL-CoA hydrolase specificity..........................................................................2
References .......................................................................................................................4
1.2.2 1.2.3 1.2.4 1.2.5
1.3
Chapter 2:
2.1
2.1.1
2.2
2.2.1
Characterization of YDR036C ..............................................................................10
Introduction ...................................................................................................................10
YDR036C and HIBYL-CoA hydrolase.................................................................10
Materials and Methods ..................................................................................................11
Materials and cell lines..........................................................................................11 Cloning and mutagenesis.......................................................................................11 Protein over-expression and purification...............................................................12 Assay of hydrolase activity ...................................................................................13 pH profile of YDR036C and variants....................................................................13 Modeling of YDR036C .........................................................................................13 Docking of substrates ............................................................................................13
Results ...........................................................................................................................14
Sequence alignment & homology .........................................................................14 Hydrolase activities ...............................................................................................14
2.2.2 2.2.3 2.2.4 2.2.5 2.2.6 2.2.7
2.3
2.3.1 2.3.2
- ""!
- !
2.3.3 2.3.4 2.3.5
Modeling and docking...........................................................................................15 Residues controlling methyl group preference......................................................15 Residues controlling hydroxyl group and carbon chain requirement....................16
Discussion......................................................................................................................17 References .....................................................................................................................19
2.4 2.5
Chapter 3:
3.1
3.1.1
Phosphorylation of HIBYL-CoA hydrolase..........................................................29
Introduction ...................................................................................................................29
HIBYL-CoA hydrolase regulation ........................................................................29 Mitochondrial regulation by phosphorylation.......................................................29 Determination of phosphorylation state ................................................................29
Materials and Methods ..................................................................................................29
Cloning and mutagenesis.......................................................................................29 Assay of hydrolase activity ...................................................................................30
Results and Discussion..................................................................................................30
Sequence alignment of HIBYL-CoA hydrolases ..................................................30 Phosphorylation mimics ........................................................................................30 Future work ...........................................................................................................31
References .....................................................................................................................32
3.1.2 3.1.3
3.2
3.2.1 3.2.2
3.3
3.3.1 3.3.2 3.3.3
3.4
Chapter 4:
4.1
4.1.1
Potential role of YDR036C in regulating ergosterol metabolism .........................38
Introduction ...................................................................................................................38
YDR036C homology to valine hydrolase .............................................................38 Hydrolase activity..................................................................................................38 Homology modeling..............................................................................................38 Ergosterol ..............................................................................................................39 Synthesis of ergosterol in the ER ..........................................................................39
4.1.2 4.1.3 4.1.4 4.1.5
- """!
- !
4.1.6 4.1.7 4.1.8 4.1.9
Shuttling of ergosterol to the LP ...........................................................................40 Shuttling of ergosterol to the PM ..........................................................................40 Detoxification by Atf2 and Say1...........................................................................40 Hydrolysis of sterol esters .....................................................................................40
Methods and Materials ..................................................................................................41
Materials and cell lines..........................................................................................41 Modelling of YDR036C........................................................................................41 Docking of substrates ............................................................................................42 Extraction and quantification of ergosterol ...........................................................42
Results and Discussion..................................................................................................42
Saccharomyces cerevisiae ydr036c !.....................................................................43 Ergosterol content of ydr036c knockout S. cerevisiae ..........................................43 Other evidence for a role for YDR036C in mitochondrial ergosterol regulation..44
Conclusions ...................................................................................................................44
Ergosterol conclusions...........................................................................................44 Implication for higher eukaryotes .........................................................................45 Future work ...........................................................................................................45
References .....................................................................................................................47
4.2
4.2.1 4.2.2 4.2.3 4.2.4
4.3
4.3.1 4.3.2 4.3.3
4.4
4.4.1 4.4.2 4.4.3
4.5
Chapter 5:
5.1 YDR036C and other yeast hydrolases...........................................................................56
Conclusions ...........................................................................................................56
- "#!
- !
!
List of Tables
Table 2.1 Table 2.2
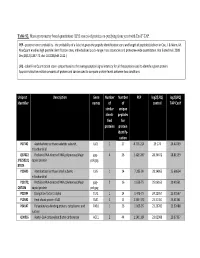
The kinetic parameters of the site-directed mutants of human and S. cerevisiae HIBYL-CoA hydrolases.…..………………………………………………….…21
The kcat and turnover ratio of human, A. thaliana, S. cerevisiae, S. pombe, C.
albicans, and D. hansenii HIBYL-CoA hydrolases.………………...…………...22
Table 2.3 Table 2.4
The kcat of S. cerevisiae wild-type and truncated HIBYL-CoA hydrolases..........23 The kcat of S. cerevisiae wild-type and glutamate 121 mutants of HIBYL-CoA hydrolase. …………………………………………………………………….….24
- Table 3.1
- The kcat of human and S. cerevisiae wild-type and mutant hydrolases….….……37
- #
- !
List of Figures
Figure 1.1 Figure 1.2 Figure 1.3 Figure 1.4 Figure 1.5 Figure 2.1
Secondary structure of the crotonase super-family core…………………………..5 Crystal structure of crotonase (2DUB) and HIBYL-CoA hydrolase (3BPT).........6 A clustalw [15] alignment of several members of the crotonase super-family…...7 Mechanism of HIBYL-CoA hydrolase. ………………………………………….8 Catabolic pathway for the branched chain amino acids………..………………....9 The proposed mechanism of 3-HP-CoA hydrolysis based on the mechanism demonstrated for Pseudomonas HIBYL-CoA hydrolase………………………...25
Figure 2.2
The clustalw alignment of HIBYL-CoA hydrolases from A. thaliana. H. sapiens. S. pombe. C. albicans. D. hansenii. S. cerevisiae. C. glabrata. and K. lactis……26
Figure 2.3 Figure 2.4
The modeled structure of the S. cerevisiae hydrolase, YDR036C….……………27 The Michaelis-Menten kinetic graphs of human and S. cerevisae HIBYL-CoA hydrolases………………………………………………………………………...28
Figure 3.1 Figure 3.2
Catabolic pathway of the branched chain amino acids…………………………..33 The clustalw alignment of HIBYL-CoA hydrolases from A. thaliana. H. sapiens.
S. pombe. C. albicans. D. hansenii. S. cerevisiae. C. glabrata. and K. lactis……34
Figure 3.3 Figure 4.1
Structure of HIBYL-CoA hydrolase……………………………………………..35 Structures of quercetin and (3'(N-carboxymethyl)carbomyl-3,4',5,7- tetrahydroxyflavone) (QC12)…………………………………………….............50
Figure 4.2 Figure 4.3
Overlay of ergosterol and quercetin in the YDR036C active site………………..51 Structures of sterols (ergosterol, cholesterol, and lanosterol), and the steroid pregnenolone……………………………………………………………………..52
Figure 4.4
Figure 4.5
The structures of coenzyme A and the truncated 3-HP-CoA used for docking with AutoDock4……………………………………………………………………….53
The ergosterol content of wild-type and ydr036c ! strains of S. cerevisiae in YPD and the growth of wild-type and ydr036c ! strains on YPD agar containing the antifungal compound clorimazole……………………………………………......54
- #"!
- !
- Figure 4.6
- Proposed sterol trafficking in S. cerevisiae………………………………………55
- #""!
- !
Dedication
First and foremost, I would like to dedicate this work to my wife, my love. If not for her continual support I may have lost my way. Second I would like to honor John Hawes for his guidance and friendship during my graduate career and beyond. He will be sorely missed. Lastly, I would like to thank the members of my committee, especially Dr. Ann Hagerman and Dr. Chris Makaroff, who have guided me in John’s absence.
- #"""
- !
Acknowledgments
I would like to acknowledge the Chemistry Department at Miami University for providing an excellent training in biochemistry, and how to succeed after graduate school. I would also like to acknowledge Dr. Richard Taylor, Dr. Gary Lorigan, and Dr. Paul James for serving on my committee.
- "$
- !
- Chapter 1:
- Introduction
1.1 Crotonase 1.1.1 Crotonase The crotonase super-family is one of the largest and arguably most catalytically diverse groups of enzymes [1-3]. The first enzyme of its type to be characterized was enoyl-CoA hydratase, also known as crotonase, which lends its name to the super-family [4, 5]. Since the initial characterization of crotonase many other members of the super-family have been added based on their sequence homology. The structure of crotonase was experimentally determined in 1996 [6] and revealed the structures of the canonical fold and oxyanion hole which are common to all super-family members.
The canonical fold is generated by repeating !!" units as seen in Figure 1.1 and 1.2 [2]. The units form two beta sheets that are approximately perpendicular to one another (colored blue in Figure 1.2) generating the scaffolding of the active site and CoA recognition site [7, 8]. The oxyanion hole is a hallmark of enzyme chemistry that accelerates carbonyl chemistry by the stabilization of enolate or other unstable anionic intermediates. Stabilization of the oxyanion, a negatively charged oxygen alpha to a double bond, is typically achieved by hydrogen bonding. The dipole-dipole interaction of the oxyanion hole is sufficient to stabilize intermediates that otherwise would be highly unfavorable. An example of this stabilization is the oxyanion hole in crotonase (colored yellow in Figure 1.2A and B) [6], where amide hydrogens of the peptide backbone stabilize the oxyanion.
The crotonase super-family enzymes generally utilize similar chemistries to perform their reactions owing their catalytic efficacy to both the oxyanion hole and the energy provided by the CoA substrates. Most crotonase family enzymes utilize acyl-CoAs as substrates. Not surprisingly, the areas of greatest conservation among the super-family are within the CoA binding regions, oxyanion hole, and to a lesser extent the residues that participate in the catalysis, while the overall sequence similarity is low, often less than 20% [1] (Figure 1.3).
- 1.2
- !-hydroxyisobutyryl-CoA hydrolase
1.2.1 HIBYL-CoA hydrolase homology Hydroxyisobutyryl-CoA hydrolase (HIBYL-CoA hydrolase) is a crotonase super-family enzyme that catalyzes the hydrolysis of hydroxyisobutyryl-CoA to hydroxybutyrate and free CoA (Figure 1.4). HIBYL-CoA hydrolase is ubiquitous throughout prokaryotes and eukaryotes, where it is believed to aid in the removal of a toxic intermediate of valine metabolism, methacrylyl-CoA [9]. Similar to the rest of the crotonase super-family, HIBYL-CoA hydrolase homologues do not
1!
!!
exhibit high similarity over the entire sequence, but the residues in key structural features required for catalysis are conserved.
1.2.2 HIBYL-CoA hydrolase and valine metabolism A subset of amino acids is known as the branched chain amino acids, which include valine, leucine and isoleucine. All three branched chain amino acids have similar hydrophobic properties that are critical for the proper formation of most globular proteins. The similar structures allow the amino acids to share the initial steps of their degradation before diverging to accommodate each individual amino acid (Figure 1.5) [10]. The common pathway consists of deamination followed by the removal of a carboxylate, and then dehydrogenation to yield a beta unsaturated acyl-CoA. The pathways all maintain a CoA ester throughout degradation except for valine. The valine pathway breaks the CoA ester bond (step 8, Figure 1.5) only to regenerate a new CoA ester bond in a subsequent step. The seemingly wasted energy of breaking and reforming the CoA ester is believed to function as a protective mechanism to prevent the buildup of methacrylyl-CoA [11]. Methacrylyl-CoA is a good Michael addition acceptor that is capable of modifying thiol groups such as those found in glutathione, free CoA, and cysteines in the active sites of the cysteine proteases. Methacrylyl-CoA represents a significant danger to the cell if allowed to accumulate because of its reactivity. The reaction catalyzed by HIBYL-CoA hydrolase (step 8, Figure 1.5), unlike most of the enzymes in valine degradation, is not reversible and therefore can behave as a one-way valve to ensure the continued progression of intermediates in the valine degradation pathway and prevent methacrylyl-CoA accumulation.
1.2.3 HIBYL-CoA hydrolase structure HIBYL-CoA hydrolase bears only minor differences from other members of the crotonase superfamily, with the most significant difference being the addition of a C-terminus whose secondary structure forms a cap over the crotonase active site groove (C-terminal is orange in Figure 1.2). The capping of the active site groove effectively prevents the catalysis of long chain acyl-CoAs generating the selectivity of HIBYL-CoA hydrolase for smaller acyl- chains. The active site is comprised of two amide hydrogens forming the oxyanion hole and an acidic residue to perform catalysis [3].
1.2.4 HIBYL-CoA hydrolase mechanism HIBYL-CoA hydrolase has a different mechanism than many other enzymes of the crotonase super-family [3]. While many crotonases have an enolate intermediate that is stabilized by the oxyanion hole, HIBYL-CoA hydrolase forms an enzyme-bound anhydride that is stabilized by the oxyanion hole (Figure 1.4). Hydrolysis is initiated by attack of a glutamate residue on the carbonyl oxygen of the CoA thioester, with formation of a tetrahedral intermediate. The intermediate collapses to the glutamyl anhydride upon release of free Coenzyme A. Subsequent
2!
!!
attack by water on either the substrate carboyl (shown in Figure 1.4) or the glutamyl carbonyl (not shown) releases the free HIBA and restores the active site glutamate.
1.2.5 HIBYL-CoA hydrolase specificity The activity of the hydrolase is specific for HIBYL-CoA and 3-hydroxypropionyl-CoA, but not equally toward both, as the enzyme has 3-fold lower activity towards 3-hydroxypropional-CoA. Both of these substrates share the common features of a three-carbon backbone and a hydroxyl group at the beta position [12, 13]. A variety of other CoAs have been assayed with HIBYL- CoA hydrolases, including substrates that contain hydroxyl groups at positions other than the beta position as well as carbon backbones other than 3 carbons, but the enzyme does not demonstrate significant activity towards other compounds.