Table S2. Mass Spectrometry Based Quantitation (LFQ Scores) of Proteins Co-Purifying from Yeast with Exoy-TAP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

C8cc08685k1.Pdf
Electronic Supplementary Material (ESI) for ChemComm. This journal is © The Royal Society of Chemistry 2018 Supporting Information Minimalist Linkers Suitable for Irreversible Inhibitors in Simultaneous Proteome Profiling, Live-Cell Imaging and Drug Screening Cuiping Guo,Yu Chang, Xin Wang, Chengqian Zhang, Piliang Hao*, Ke Ding and Zhengqiu Li* School of Pharmacy, Jinan University, Guangzhou, China 510632 *Corresponding author ([email protected]) 1. General Information All chemicals were purchased from commercial vendors and used without further purification, unless indicated otherwise. All reactions requiring anhydrous conditions were carried out under argon or nitrogen atmosphere using oven-dried glassware. AR-grade solvents were used for all reactions. Reaction progress was monitored by TLC on pre-coated silica plates (Merck 60 F254 nm, 0.25 µm) and spots were visualized by UV, iodine or other suitable stains. Flash column chromatography was carried out using silica gel (Qingdao Ocean). All NMR spectra (1H-NMR, 13C-NMR) were recorded on Bruker 300 MHz/400 MHz NMR spectrometers. Chemical shifts were reported in parts per million (ppm) referenced with respect to appropriate internal standards or residual solvent peaks (CDCl3 = 7.26 ppm, DMSO-d6 = 2.50 ppm). The following abbreviations were used in reporting spectra, br s (broad singlet), s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), dd (doublet of doublets). Mass spectra were obtained on Agilent LC-ESI-MS system. All analytical HPLC were carried out on Agilent system. Water with 0.1% TFA and acetonitrile with 0.1% TFA were used as eluents and the flow rate was 0.5 mL/min. -

The Rise and Fall of the Bovine Corpus Luteum
University of Nebraska Medical Center DigitalCommons@UNMC Theses & Dissertations Graduate Studies Spring 5-6-2017 The Rise and Fall of the Bovine Corpus Luteum Heather Talbott University of Nebraska Medical Center Follow this and additional works at: https://digitalcommons.unmc.edu/etd Part of the Biochemistry Commons, Molecular Biology Commons, and the Obstetrics and Gynecology Commons Recommended Citation Talbott, Heather, "The Rise and Fall of the Bovine Corpus Luteum" (2017). Theses & Dissertations. 207. https://digitalcommons.unmc.edu/etd/207 This Dissertation is brought to you for free and open access by the Graduate Studies at DigitalCommons@UNMC. It has been accepted for inclusion in Theses & Dissertations by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. THE RISE AND FALL OF THE BOVINE CORPUS LUTEUM by Heather Talbott A DISSERTATION Presented to the Faculty of the University of Nebraska Graduate College in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Biochemistry and Molecular Biology Graduate Program Under the Supervision of Professor John S. Davis University of Nebraska Medical Center Omaha, Nebraska May, 2017 Supervisory Committee: Carol A. Casey, Ph.D. Andrea S. Cupp, Ph.D. Parmender P. Mehta, Ph.D. Justin L. Mott, Ph.D. i ACKNOWLEDGEMENTS This dissertation was supported by the Agriculture and Food Research Initiative from the USDA National Institute of Food and Agriculture (NIFA) Pre-doctoral award; University of Nebraska Medical Center Graduate Student Assistantship; University of Nebraska Medical Center Exceptional Incoming Graduate Student Award; the VA Nebraska-Western Iowa Health Care System Department of Veterans Affairs; and The Olson Center for Women’s Health, Department of Obstetrics and Gynecology, Nebraska Medical Center. -

Reprogramming of Trna Modifications Controls the Oxidative Stress Response by Codon-Biased Translation of Proteins
Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Chan, Clement T.Y. et al. “Reprogramming of tRNA Modifications Controls the Oxidative Stress Response by Codon-biased Translation of Proteins.” Nature Communications 3 (2012): 937. As Published http://dx.doi.org/10.1038/ncomms1938 Publisher Nature Publishing Group Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/76775 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins Clement T.Y. Chan,1,2 Yan Ling Joy Pang,1 Wenjun Deng,1 I. Ramesh Babu,1 Madhu Dyavaiah,3 Thomas J. Begley3 and Peter C. Dedon1,4* 1Department of Biological Engineering, 2Department of Chemistry and 4Center for Environmental Health Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139; 3College of Nanoscale Science and Engineering, University at Albany, SUNY, Albany, NY 12203 * Corresponding author: PCD, Department of Biological Engineering, NE47-277, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139; tel 617-253-8017; fax 617-324-7554; email [email protected] 2 ABSTRACT Selective translation of survival proteins is an important facet of cellular stress response. We recently demonstrated that this translational control involves a stress-specific reprogramming of modified ribonucleosides in tRNA. -

Allele-Specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish
Allele-specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish by Ailu Chen A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama August 1, 2015 Keywords: catfish, interspecific hybrids, allele-specific expression, ribosomal protein Copyright 2015 by Ailu Chen Approved by Zhanjiang Liu, Chair, Professor, School of Fisheries, Aquaculture and Aquatic Sciences Nannan Liu, Professor, Entomology and Plant Pathology Eric Peatman, Associate Professor, School of Fisheries, Aquaculture and Aquatic Sciences Aaron M. Rashotte, Associate Professor, Biological Sciences Abstract Interspecific hybridization results in a vast reservoir of allelic variations, which may potentially contribute to phenotypical enhancement in the hybrids. Whether the allelic variations are related to the downstream phenotypic differences of interspecific hybrid is still an open question. The recently developed genome-wide allele-specific approaches that harness high- throughput sequencing technology allow direct quantification of allelic variations and gene expression patterns. In this work, I investigated allele-specific expression (ASE) pattern using RNA-Seq datasets generated from interspecific catfish hybrids. The objective of the study is to determine the ASE genes and pathways in which they are involved. Specifically, my study investigated ASE-SNPs, ASE-genes, parent-of-origins of ASE allele and how ASE would possibly contribute to heterosis. My data showed that ASE was operating in the interspecific catfish system. Of the 66,251 and 177,841 SNPs identified from the datasets of the liver and gill, 5,420 (8.2%) and 13,390 (7.5%) SNPs were identified as significant ASE-SNPs, respectively. -

Supplemental Data
SUPPLEMENTAL INFORMATION Glomerular cell crosstalk influences composition and assembly of extracellular matrix Adam Byron,1,*,† Michael J. Randles,1,2,† Jonathan D. Humphries,1 Aleksandr Mironov,1 Hellyeh Hamidi,1 Shelley Harris,2 Peter W. Mathieson,3 Moin A. Saleem,3 Simon S. Satchell,3 Roy Zent,4,5 Martin J. Humphries,1 and Rachel Lennon.1,2 1Wellcome Trust Centre for Cell-Matrix Research, Faculty of Life Sciences, University of Manchester, Manchester, UK; 2Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK; 3Academic Renal Unit, Faculty of Medicine and Dentistry, University of Bristol, Bristol, UK; 4Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA; and 5Veterans Affairs Hospital, Nashville, TN, USA. *Present address: Edinburgh Cancer Research UK Centre, Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK. †These authors contributed equally to this work. Corresponding author: Dr Rachel Lennon, Wellcome Trust Centre for Cell-Matrix Research, Michael Smith Building, University of Manchester, Manchester M13 9PT, UK. Phone: 0044 (0) 161 2755498. Fax: 0044 (0) 161 2755082. Email: [email protected] Supplementary methods Non-glomerular cell culture HEK 293T and human foreskin fibroblasts were cultured until confluent in Dulbecco's Modified Eagle Medium supplemented with 10% foetal calf serum. Lentiviral production and transduction Podocytes stably expressing GFP were produced by lentiviral transduction. Briefly, HEK 293T cells were transfected with three plasmids obtained from Addgene (psPAX2 Addgene ID 12260, pMD2.G Addgene ID 12259 and pWPXL Addgene ID 12257) using polyethyleneimine (Sigma-Aldrich). Conditioned medium containing viruses was collected after 5 days following several media changes including an 8 hr incubation with sodium butyrate- containing media to promote virus production. -

Supplementary Materials
1 Supplementary Materials: Supplemental Figure 1. Gene expression profiles of kidneys in the Fcgr2b-/- and Fcgr2b-/-. Stinggt/gt mice. (A) A heat map of microarray data show the genes that significantly changed up to 2 fold compared between Fcgr2b-/- and Fcgr2b-/-. Stinggt/gt mice (N=4 mice per group; p<0.05). Data show in log2 (sample/wild-type). 2 Supplemental Figure 2. Sting signaling is essential for immuno-phenotypes of the Fcgr2b-/-lupus mice. (A-C) Flow cytometry analysis of splenocytes isolated from wild-type, Fcgr2b-/- and Fcgr2b-/-. Stinggt/gt mice at the age of 6-7 months (N= 13-14 per group). Data shown in the percentage of (A) CD4+ ICOS+ cells, (B) B220+ I-Ab+ cells and (C) CD138+ cells. Data show as mean ± SEM (*p < 0.05, **p<0.01 and ***p<0.001). 3 Supplemental Figure 3. Phenotypes of Sting activated dendritic cells. (A) Representative of western blot analysis from immunoprecipitation with Sting of Fcgr2b-/- mice (N= 4). The band was shown in STING protein of activated BMDC with DMXAA at 0, 3 and 6 hr. and phosphorylation of STING at Ser357. (B) Mass spectra of phosphorylation of STING at Ser357 of activated BMDC from Fcgr2b-/- mice after stimulated with DMXAA for 3 hour and followed by immunoprecipitation with STING. (C) Sting-activated BMDC were co-cultured with LYN inhibitor PP2 and analyzed by flow cytometry, which showed the mean fluorescence intensity (MFI) of IAb expressing DC (N = 3 mice per group). 4 Supplemental Table 1. Lists of up and down of regulated proteins Accession No. -

Swissprot Genetrail Acc. No. ID Exp1 Exp2 Exp1 Exp2 Exp1 Exp2
Supplementary TABLE 1. Quantitative MS-based proteomic results from the macrophage cytosolic proteomes for both biological replicates separately Human proteins HSV-1 dLeu d3-4 Protein name SwissProt Genetrail N % Seq Cov Pept(95%) Fold change P-value Fold change P-value Fold change P-value Acc. No. ID Exp1 Exp2 Exp1 Exp2 Exp1 Exp2 Exp1 Exp2 Exp1 Exp2 Exp1 Exp2 Exp1 Exp2 Exp1 Exp2 Exp1 Exp2 10 kDa heat shock protein, mitochondrial P61604 HSPE1 121 108 69,6 65,7 9 18 1,24 1,11 0,014 0,141 1,12 1,16 0,127 0,054 1,07 0,96 0,290 0,529 14-3-3 protein beta/alpha P31946 YWHAB 350 145 37 52,9 13 16 1,10 0,97 0,409 0,743 1,12 0,72 0,651 0,184 1,04 0,72 0,730 0,101 14-3-3 protein epsilon P62258 YWHAE 661 524 16,5 24,7 5 8 1,43 1,00 0,996 0,60 0,78 0,238 0,84 0,87 0,765 14-3-3 protein eta Q04917 YWHAH 572 376 36,2 34,6 9 10 0,77 1,00 1,30 14-3-3 protein gamma P61981 YWHAG 135 358 38,1 35,2 13 10 1,93 1,21 0,170 1,10 0,44 0,515 1,88 1,17 0,099 14-3-3 protein theta P27348 YWHAQ 659 596 20,4 43,7 7 8 1,12 0,72 1,16 14-3-3 protein zeta/delta P63104 YWHAZ 59 36 45,3 54,7 15 19 1,48 1,33 0,004 0,019 1,22 0,95 0,265 0,570 1,08 1,10 0,315 0,416 182 kDa tankyrase-1-binding protein Q9C0C2 TNKS1BP1 517 2,7 2 0,89 0,874 1,18 0,345 1,06 0,651 1-phosphatidylinositol 4,5-bisphosphate phosphodiesteraseQ00722 beta-2 PLCB2 291 9,1 3 0,67 0,290 0,65 0,046 0,94 0,793 2,4-dienoyl-CoA reductase, mitochondrial Q16698 DECR1 335 14,3 4 0,76 0,284 0,97 0,841 0,91 0,415 26S proteasome non-ATPase regulatory subunit 11 O00231 PSMD11 375 18,7 3 1,43 1,19 0,93 26S proteasome non-ATPase -

Figure S1. Quality Control Validation of MS Data. (A‑C) Mass Error Distribution of All Peptides Identified in the Acetylome
Figure S1. Quality control validation of MS data. (A‑C) Mass error distribution of all peptides identified in the acetylome, succi- nylome and quantitative proteome, respectively. (D‑F) Length distribution of peptides identified in the acetylome, succinylome and quantitative proteome, respectively. Figure S2. Comparison of modification level between breast cancer tissue and normal tissue. Comparison of acetylation level (A) and succinylation level (B) between breast cancer tissue and normal tissue. Data are medians and were analyzed using Wilcoxon Signed Rank Test. **P<0.01. Table SI. Protein sites whose acetylation and succinylation levels were both significantly upregulated in breast cancer tissues (fold change ≥1.5 compared with normal tissues). Protein ID Protein name Modification site P54868 HMCS2 310K Q15063 POSTN 549K Q99715 COCA1 1601K P51572 BAP31 72K P07237 PDLA1 328K Q06830 PRDX1 192K P48735 IDHP 180K P30101 PDIA3 417K P0DMV9 HS71B 526K Q01995 TAGL 21K P06748 NPM1 27K Q00325 MPCP 209K P00488 F13A 69K P02545 LMNA 260K P08133 ANXA6 478K P02452 CO1A1 751K Table SII. Protein sites whose acetylation and succinylation levels were both significantly downregulated in breast cancer tissues (fold change ≥1.5 compared with normal tissues). Protein ID Protein name Modification site RET4 P02753 30K PSG2 P07585 142K HBA P69905 12K IGKC P01834 80K HBA P69905 8K Table SIII. All proteins whose expression level were significantly upregulated in breast cancer tissues (fold change ≥1.5 compared with normal tissues). Protein ID Protein description -

Immunoprecipitation and Mass Spectrometry Defines an Extensive
BRES : 44759 Model7 pp: À 1221ðcol:fig: : NILÞ brain research ] ( ]]]]) ]]]– ]]] Available online at www.sciencedirect.com 121 122 123 124 125 126 www.elsevier.com/locate/brainres 127 128 129 Review 130 131 fi 132 Immunoprecipitation and mass spectrometry de nes 133 – 134 an extensive RBM45 protein protein interaction 135 Q2 136 network 137 138 a a,b a a c 139 Yang Li , Mahlon Collins , Jiyan An , Rachel Geiser , Tony Tegeler , c c c a,b,n 140 Q1 Kristine Tsantilas , Krystine Garcia , Patrick Pirrotte , Robert Bowser 141 aDivisions of Neurology and Neurobiology, Barrow Neurological Institute, St. Joseph's Hospital and Medical Center, 142 Phoenix, AZ 85013, USA 143 bUniversity of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA 144 cCenter for Proteomics, TGen (Translational Genomics Research Institute), Phoenix, AZ 85004, USA 145 146 147 article info abstract 148 149 Article history: The pathological accumulation of RNA-binding proteins (RBPs) within inclusion bodies is a 150 Received 30 January 2016 hallmark of amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration 151 Received in revised form (FTLD). RBP aggregation results in both toxic gain and loss of normal function. Determining 152 25 February 2016 the protein binding partners and normal functions of disease-associated RBPs is necessary 153 Accepted 28 February 2016 to fully understand molecular mechanisms of RBPs in disease. Herein, we characterized 154 the protein–protein interactions (PPIs) of RBM45, a RBP that localizes to inclusions in ALS/ 155 – fi Keywords: FTLD. Using immunoprecipitation coupled to mass spectrometry (IP MS), we identi ed 132 156 fi RBM45 proteins that speci cally interact with RBM45 within HEK293 cells. -

Allosteric Integrase Inhibitor Potency Is Determined Through the Inhibition of HIV-1 Particle Maturation
Allosteric integrase inhibitor potency is determined through the inhibition of HIV-1 particle maturation Kellie A. Juradoa, Hao Wanga, Alison Slaughterb, Lei Fengb, Jacques J. Kesslb, Yasuhiro Koha, Weifeng Wanga, Allison Ballandras-Colasa, Pratiq A. Patelc, James R. Fuchsc, Mamuka Kvaratskheliab, and Alan Engelmana,1 aDepartment of Cancer Immunology and AIDS, Dana-Farber Cancer Institute and Department of Medicine, Harvard Medical School, Boston, MA 02215; and bCenter for Retrovirus Research and Comprehensive Cancer Center and cDivision of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, The Ohio State University, Columbus, OH 43210 Edited by Alan R. Rein, National Cancer Institute, Frederick, MD, and accepted by the Editorial Board April 1, 2013 (received for review January 14, 2013) Integration is essential for HIV-1 replication, and the viral integrase HIV-1 preferentially integrates along the bodies of active genes (IN) protein is an important therapeutic target. Allosteric IN inhib- (6), a trait that is largely attributable to an interaction between itors (ALLINIs) that engage the IN dimer interface at the binding site IN and the host protein lens epithelium-derived growth factor for the host protein lens epithelium-derived growth factor (LEDGF)/ (LEDGF)/transcriptional coactivator p75 (reviewed in refs. 7 and transcriptional coactivator p75 are an emerging class of small mole- 8). LEDGF/p75 functions as a bimodal tether during integration: cule antagonists. Consistent with the inhibition of a multivalent drug elements within its N-terminal region confer constitutive binding to target, ALLINIs display steep antiviral dose–response curves ex vivo. chromatin, whereas a downstream IN-binding domain (IBD) binds ALLINIs multimerize IN protein and concordantly block its assembly lentiviral IN proteins (9, 10). -

Profilin-1 Is Required for Survival of Adult Hematopoietic Stem Cells
Extended methods Immunohistochemistry HepG-2, SMMC-7721, and 293T cells were obtained from Cell Resource Center of Shanghai Institute for Biological Science, Chinese Academy Science, Shanghai, China. HUVEC cells were kindly provided by Prof. Ping-Jin Gao at Institute of Health Sciences (Shanghai, China). All these cell lines were cultured in DMEM with 10% FBS. MDA- MB-231 cell line was kindly provided by Prof. Ming-Yao Liu (East China Normal University, Shanghai, China) and was cultured in Leibovitz L-15 medium with 10% FBS. All these cell lines were originally purchased from ATCC. MDA-MB-231, SMMC-7721 or HepG2 cells were grown on coverslips in 24-well plates and fixed in either 4% paraformaldehyde or pre-chilled methanol (-20°C) for 10 min. In some cases, WT or VPS33B-null Lin-Sca-1+c-Kit+Flk2-CD34- LT-HSCs were collected by flow cytometry and fixed for immunofluorescence staining. Cells were then blocked with 3% BSA in PBS for 60 min followed by incubation with primary antibodies overnight. The antibodies used were anti-HA (Sigma), anti-Flag (Sigma), anti-VPS33B (Sigma), anti- VPS16B (Abcam), anti-GDI2 (Proteintech), anti-LAMP1 (Proteintech), anti-FLOT1 (Abways), anti-CD63 (Proteintech), anti-ANGPTL2 (R&D system), anti-ANGPTL3 (R&D system), anti-TPO (Abways), anti-GLUT1 (Proteintech), anti-LDHA (Proteintech), anti-PKM2 (CST), anti-RAB11A (Abways), anti-RAB27A (Abways) and anti-V5 (Biodragon). Fluorescent-conjugated secondary antibodies (Alexa Fluor® 488 or Alexa Fluor® 555) against mouse, rabbit, or goat were obtained from the Thermo Scientific Inc. The details for all the antibodies are listed in Table S3. -
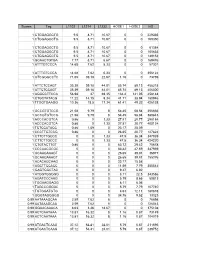
Scores Tag L1102 L1214 L1232 HOSE1 HOSE2 HS 1
Scores Tag L1102 L1214 L1232 HOSE1 HOSE2 HS 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 229335 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 169350 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 61384 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 105633 1 CTGGAGGCTG 9.5 8.71 10.67 0 0 149152 1 GCAACTGTGA 7.77 8.71 6.67 0 0 169476 1 ATTTGTCCCA 14.68 7.62 5.33 0 0 57301 1 ATTTGTCCCA 14.68 7.62 5.33 0 0 356122 1 GTCGGGCCTC 71.65 39.18 22.67 1.16 0 73769 1 ATTCTCCAGT 35.39 39.18 44.01 85.74 89.13 458218 1 ATTCTCCAGT 35.39 39.18 44.01 85.74 89.13 406300 1 AGGGCTTCCA 56.98 37 69.35 134.4 141.05 458148 1 CTGCTATACG 11.22 14.15 9.34 41.71 38.94 180946 1 TTGGTGAAGG 10.36 18.5 17.34 61.41 49.32 426138 1 GCCGTGTCCG 21.58 9.79 8 54.45 58.84 356666 1 GCCGTGTCCG 21.58 9.79 8 54.45 58.84 380843 1 ACCCACGTCA 0.86 0 1.33 27.81 20.77 298184 1 ACCCACGTCA 0.86 0 1.33 27.81 20.77 400124 1 TCTCCATACC 0.86 1.09 0 23.17 25.09 1 CCCTTGTCCG 0.86 0 0 26.65 20.77 127824 1 CTTCTTGCCC 0 0 1.33 47.5 36.34 347939 1 CTTCTTGCCC 0 0 1.33 47.5 36.34 424220 1 CTGTACTTGT 0.86 0 0 63.72 29.42 75678 1 CCCAACGCGC 0 0 0 83.42 47.59 347939 1 GCAAGAAAGT 0 0 0 26.65 39.81 36977 1 GCAAGAAAGT 0 0 0 26.65 39.81 155376 1 ACACAGCAAG 0 0 0 23.17 15.58 1 AGCTTCCACC 0 0 0 11.59 7.79 355542 1 GAGTGGCTAC 0 0 0 9.27 6.92 1 ATGGTGGGGG 0 0 0 8.11 22.5 343586 1 AGATCCCAAG 0 0 0 5.79 8.65 50813 1 TGGAAGGAGG 0 0 0 8.11 6.06 1 TAGCCGGGAC 0 0 0 5.79 7.79 107740 1 TGTGGATGTG 0 0 0 4.63 12.11 180878 1 GGGTAGGGGG 0 0 0 34.76 9.52 13323 0.99 AATAAAGCAA 2.59 7.62 8 0 0 76698 0.99 AATAAAGCAA 2.59 7.62 8 0 0 126043 0.99 GGAACAAACA 8.63 3.26 18.67 0 0 375108