Electronic Supplementary Material (ESI) for ChemComm. This journal is © The Royal Society of Chemistry 2018
Supporting Information
Minimalist Linkers Suitable for Irreversible Inhibitors in Simultaneous Proteome Profiling,
Live-Cell Imaging and Drug Screening
Cuiping Guo,Yu Chang, Xin Wang, Chengqian Zhang, Piliang Hao*, Ke Ding and Zhengqiu Li*
School of Pharmacy, Jinan University, Guangzhou, China 510632
*Corresponding author ([email protected])
1. General Information
All chemicals were purchased from commercial vendors and used without further purification, unless indicated otherwise. All reactions requiring anhydrous conditions were carried out under argon or nitrogen atmosphere using oven-dried glassware. AR-grade solvents were used for all reactions. Reaction progress was monitored by TLC on pre-coated silica plates (Merck 60 F254 nm, 0.25 µm) and spots were visualized by UV, iodine or other suitable stains. Flash column chromatography was carried out using silica gel (Qingdao Ocean). All NMR spectra (1H-NMR, 13C-NMR) were recorded on Bruker 300 MHz/400 MHz NMR spectrometers. Chemical shifts were reported in parts per million (ppm) referenced with respect to appropriate internal standards or residual solvent peaks (CDCl3 = 7.26 ppm, DMSO-d6 = 2.50 ppm). The following abbreviations were used in reporting spectra, br s (broad singlet), s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), dd (doublet of doublets). Mass spectra were obtained on Agilent LC-ESI-MS system. All analytical HPLC were carried out on Agilent system. Water with 0.1% TFA and acetonitrile with 0.1% TFA were used as eluents and the flow rate was 0.5 mL/min. Antibodies against EGFR (ab52894) and BTK (ab137503) were purchased from Cell Signaling Technology (CST). Click reagents were purchased from Click Chemistry Tools (https://clickchemistrytools.com/).
2. Cell culture and Western blot
Cell lines were obtained from the National Cancer Institute Developmental Therapeutics Program (NCI-60). Cells were cultured in Dulbeccoʼs modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) or RPMI 1640 Medium (Invitrogen, Carlsbad, CA)
containing 10% heat-inactivated fetal bovine serum (FBS; Invitrogen), 100 units/mL penicillin, and 100 μg/mL streptomycin
(Thermo Scientific) and maintained in a humidified 37 °C incubator with 5% CO2. To generate protein lysates, cells were washed twice with cold phosphate-buffered saline (PBS), harvested with 1× trypsin or by use of a cell scraper, and collected by centrifugation. Cell pellets were then washed with PBS and lysed with RIPA (Thermo Scientific™,#89900) lysis and extraction
buffer (with Pierce™ Protease Inhibitor Tablets, Thermo Scientific™, #A32955). Protein concentration was determined by Pierce™
BCA Protein Assay Kit (Thermo Scientific™, #23252) and Synergy H1 Hybrid Multi-Mode Reader (BioTek). For Western blotting
experiments, samples were resolved by SDS−polyacrylamide gels and transferred to poly membranes. Membranes were then blocked
with 3% bovine serum albumin (BSA) in TBST (0.1% Tween in Tris-buffered saline) for 1 h at room temperature. After blocking, membranes were incubated with the corresponding primary antibody for another 1 hour. After incubation, membranes were washed with TBST (4×10 min) and then incubated with an appropriate secondary antibody. Finally, blots were washed again with TBST before being developed with SuperSignal West Dura Kit (Thermo Scientific), and finally imaged with Amersham Imager 600(GE Healthcare). Cell Counting Kit-8 (CCK-8, DOJINDO, #CK04) was used for cell proliferation assay. Proteome labeling, in-gel fluorescence scanning and cellular imaging experiments were performed as previously reported.[1-4]
S1_1
Table S1. The probes used in current study
- AF-1
- AF-2
- IB-1
- IB-2
IB-3
Table S2. Structures of the reporters
- TAMRA-alkyne
- TAMRA-N3
- Biotin-alkyne
- Biotin-N3
- Tetrazine-Cy5
- Methyltetrazine-biotin
S1_2
DBCO-Cy3
3. Chemical Synthesis
Scheme S1
S2 was synthesized based on previously reported procedures from S1.[5] To a solution of compound S2 (50 mg, 0.095 mmol) in 3 mL DMF was added N-methylpropargylamine (0.0317 mL, 0.38 mmol), potassium carbonate (26 mg, 0.19 mmol) and potassium iodide (27 mg, 0.17 mmol) at 0 oC. The mixture was stirred at 40 oC for 3 h under N2 gas atomosphere and then quenched by addition of 10 mL water. The resulting mixture was extracted with ethyl acetate (3 × 20 mL), and the combined organic phase was washed with brine, dried over anhydrous Na2SO4. Upon solvent evaporation in vacuo, the residue was purified by flash column
1
(methanol:ethyl acetate = 1:10) to give product AF-1 as a yellow solid (23 mg, 48%). H NMR (300 MHz, CDCl3) δ 9.00 (s, 1H), 8.56 (s, 1H), 8.35 (s, 1H), 8.10 (s, 1H), 7.79 (dd, J = 3.0, 6.0 Hz, 1H), 7.46 (m, 1H), 7.09 (s, 1H), 7.0 (m, 1H), 6.96 (m, 1H), 6.23 (d, J = 15.0 Hz, 1H), 5.10 (s, 1H), 4.15 (d, J = 12.0 Hz, 1H), 4.02 (m, 2H), 3.90 (m, 1H), 3.37 (s, 2H), 3.28 (d, J = 6.0 Hz,2H), 2.43 (m, 1H), 3.26 (s, 3H), 2.29 (t, J = 3.0 Hz, 1H), 2.22 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 163.98, 156.98, 154.63, 150.60, 148.16, 143.95, 135.46, 127.91, 125.63, 124.17, 121.89, 120.74, 116.49, 110.68, 109.61, 108.30, 79.48, 78.24, 77.23, 73.95, 73.12, 67.41, 56.55, 45.79, 42.11, 32.84. HR-MS (m/z) [M + H]+ calcd: 510.1703; Found: 510.1666.
Scheme S2
To a stirred solution of NaN3 (6 mg, 0.095 mmol) in 3 mL DMF was added S2 (50 mg, 0.095 mmol) at 0oC. The resulting mixture was stirred for 30 min and then at room temperature for 4 h. Subsequently, 5 mL water was added and the mixture was extracted with ethyl acetate (2 × 15 mL). The combined organic phase was washed with brine and then dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by flash column (methanol:CH2Cl2 = 3:50) to give AF-2 as a white solid (37 mg,
1
80%). H NMR (400 MHz, CDCl3) δ 9.10 (s, 1H), 8.65 (s, 1H), 8.13 (s, 1H), 7.93 (dd, J = 4.0, 8.0 Hz, 1H), 7.75 (s, 1H), 7.54 (m, 1H), 7.21 (s, 1H), 7.14 (t, J = 8.0 Hz, 1H), 7.01 (dt, J = 4.0 12.0 Hz, 1H), 6.32 (dt, J = 4.0, 12.0 Hz, 1H), 5.19 (t, J = 4.0 Hz, 1H), 4.20 (d, J = 12.0 Hz, 1H), 4.14 (d, J = 4.0 Hz, 2H), 4.11 (d, J = 4.0 Hz, 1H), 4.05 (m, 1H), 3.95 (m, 1H), 2.45 (m, 1H), 2.25 (m, 1H).
S1_3
13C NMR (100 MHz, CDCl3) δ 163.29, 156.96, 154.81, 150.57, 148.34, 139.60, 128.24, 125.66, 124.43, 121.95, 121.89, 116.83,
116.61, 110.18, 109.60, 108.72, 79.70, 73.18, 67.48, 51.49, 32.98, 29.92. HR-MS (m/z) [M + H]+ calcd: 484.1295; Found: 484.1290.
Scheme S3
(IB-1). The intermediate S3 is commercially available and the cyclopropene-containing linker (S4) was synthesized based on previously published procedures.[6] To a solution of compound S4 (20.0 mg, 0.05 mmol) in 4 mL DMF was added HOBT (10.49 mg, 0.075 mmol), EDC (14.37 mg, 0.075 mmol), TEA (10 mg, 0.1 mmol) and S8 (6.2 mg, 0.05 mmol), successively. The reaction was stirred at room temperature overnight prior to addition of 3 mL water and then extracted with ethyl acetate (2 × 10 mL), the combined organic phase was washed with brine, dried over anhydrous Na2SO4 and concentrated. The residue was purified by flash column (methanol:CH2Cl2 = 1 : 50) to give IB-1 as a light yellow solid (12.3 mg, 50 %). 1H NMR (300 MHz, CDCl3) δ 8.34 (s, 1H), 7.62 (d, J = 12.0 Hz, 2H), 7.37 (t, J = 6.0, 9.0 Hz, 2H), 7.14 (t, J = 9.0 Hz, 3H), 7.06 (d, J = 6.0 Hz, 2H), 6.53 (m, 1H), 6.32 (d, J = 3.0 Hz, 1H), 5.66 (s, 1H), 4.84 (m, 1H), 4.13 (d, J = 6.0 Hz, 1H), 3.67 (d, J = 6.0 Hz, 1H), 3.31 (m, 1H), 2.24 (m, 3H), 2.11 (s, 3H),
1.95 (m, 2H), 1.69 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 166.07, 158.53, 157.84, 156.35, 156.18, 155.79, 154.25, 143.83, 129.97,
127.75, 124.05, 119.54, 119.30, 119.14, 115.54, 101.24, 98.59, 53.62, 52.70, 49.87, 46.16, 45.72, 42.13, 35.96, 31.91, 30.38, 29.26, 27.22, 22.46. ESI-MS (m/z) [M + H]+ calcd: 493.2; Found: 493.5. HR-MS (m/z) [M + H]+ calcd: 493.2347; Found: 493.2331.
Scheme S4
(IB-2). Synthesis of S5 was based on previously published procedures from commercially available intermediate S3.[7] To a solution of 2 mL TFA and 6 mL DCM was added compound S5 (17.1 mg, 0.03 mmol), the reaction was then stirred at room temperature for 30 minutes. Upon solvent evaporation in vacuo, the crude product was dissolved in 3 mL DMF followed by addition of TCO-NHS (5.3 mg, 0.02 mmol) and TEA (10 mg, 0.1 mmol), the resulting mixture was stirred at room temperature for 1 hour and then quenched by addition of 3 mL water. Upon extraction with ethyl acetate (2 × 10 mL) and concentration in vacuo, the residue
1
was purified by flash column (MeOH:DCM = 1:20) to afford IB-2 (3 mg, 16% yield). H NMR (400 MHz, CDCl3) δ 8.37 (d, J = 16.4 Hz, 1H), 7.63 (d, J = 8.2 Hz, 2H), 7.40 (dd, J = 8.5, 7.4 Hz, 2H), 7.21 – 7.13 (m, 3H), 7.11 – 7.06 (m, 2H), 6.75 (s, 1H), 6.37 (t, J = 18.0 Hz, 1H), 5.88 (d, J = 65.7 Hz, 1H), 5.51 (s, 2H), 4.80 (d, J = 42.7 Hz, 2H), 4.56 (d, J = 12.9 Hz, 1H), 4.34 (d, J = 25.6 Hz, 1H), 4.15 (d, J = 11.6 Hz, 1H), 3.92 (d, J = 22.3 Hz, 3H), 3.74 (s, 1H), 3.35 (t, J = 12.0 Hz, 1H), 3.15 (d, J = 14.8 Hz, 1H), 2.87 (s, 1H), 2.31 (d, J = 30.9 Hz, 4H), 2.04 – 1.81 (m, 12H). 13C NMR (101 MHz,CDCl3) δ 158.77 , 156.22 , 134.90 , 133.00 , 130.01 , 129.92 , 127.31 , 124.17 , 119.63 , 119.15 , 41.08 , 41.00 , 38.65 , 34.26 , 32.49 , 31.92 , 30.91 , 29.79 , 29.71 , 29.61 , 29.33 , 29.25 , 27.22 , 25.55 , 22.70 , 14.14 .HR-MS (m/z) [M + H]+ calcd: 622.3136; Found: 622.3126.
S1_4
Scheme S5
(S6). To 30 mL DCM was added tert-butyl piperidin-4-ylcarbamate (400 mg, 2 mmol) and K2CO3 (552.8 mg, 4 mmol), the resulting mixture was stirred for 1 hour followed by addition of methyl (E)-4-bromobut-2-enoate (254 mg, 2 mmol) and further stirred at 60℃ for 20 hours. Upon solvent evaporation, the residue was purified by flash column to afford S6 (238.5 mg, 80% yield). 1H NMR (400 MHz, DMSO-d6) δ 6.87 – 6.70 (m, 2H), 5.98 (dt, J = 15.7, 1.6 Hz, 1H), 3.65 (s, 3H), 3.18 (dd, J = 7.5, 3.7 Hz, 1H), 3.08 (dd, J = 5.9, 1.7 Hz, 2H), 2.72 (dt, J = 11.8, 3.7 Hz, 2H), 1.95 (td, J = 12.0, 2.8 Hz, 2H), 1.67 (dd, J = 12.7, 4.2 Hz, 2H), 1.37 (s, 9H).
(S7). To a stirred solution of S6 (149 mg, 0.5 mol) in 10 mL MeOH was added 2 mL NaOH solution (2N). The reaction was stirred overnight at room temperature and then diluted with 10 mL H2O. The mixture was then acidified with 1N HCl followed by extraction with ethyl acetate (3 × 10 mL). The combined organic phase was washed with brine, dried over anhydrous Na2SO4 and concentrated. The residue was purified by flash column to give a crude product (127.8 mg, 90 %.), which can be used in next step directly. 1H NMR (400 MHz, Methanol-d4) δ 6.86 (dd, J = 15.2, 7.3 Hz, 1H), 6.20 (d, J = 15.4 Hz, 1H), 3.88 (d, J = 6.9 Hz, 2H), 3.59 (s, 1H), 3.49 – 3.35 (m, 2H), 3.17 – 3.01 (m, 2H), 2.14 – 1.98 (m, 2H), 1.85 – 1.68 (m, 2H), 1.37 (s, 9H).
To 10 mL DCM was added the crude product (99 mg, 0.35 mmol), HATU (160 mg, 0.42 mmol), TEA (70 mg, 0.7 mmol) and
S3 (135 mg, 0.35 mmol), successively. The reaction was stirred for 10 hours and then quenched by addition of 5 mL water, the resulting mixture was extracted with EtOAc (3×10 mL). The combined organics were washed with 5% NaHCO3 (2×10 mL), dried
1
over anhydrous Na2SO4, concentrated in vacuo to give compound S7 (137 mg, 60%), which can be used in next step directly. H NMR (400 MHz, Methanol-d4) δ 8.26 (d, J = 9.4 Hz, 1H), 7.71 – 7.64 (m, 2H), 7.45 – 7.37 (m, 2H), 7.22 – 7.04 (m, 5H), 6.93 – 6.57 (m, 2H), 4.58 (dd, J = 12.9, 4.1 Hz, 1H), 4.21 – 4.02 (m, 2H), 3.95 (dd, J = 13.7, 8.5 Hz, 1H), 3.69 – 3.61 (m, 1H), 3.61 – 3.40 (m, 3H), 3.19 – 3.06 (m, 1H), 2.69 (d, J = 56.6 Hz, 2H), 2.36 (dt, J = 16.1, 6.5 Hz, 1H), 2.22 (dt, J = 13.3, 4.6 Hz, 1H), 2.18 – 2.06 (m, 1H), 1.99 (dd, J = 27.9, 11.4 Hz, 2H), 1.80 – 1.54 (m, 3H), 1.44 (d, J = 4.4 Hz, 9H).
(IB-3). To a solution of 2 mL TFA and 6 mL DCM was added S7 (19.6 mg, 0.03 mmol), the reaction was stirred at room temperature for 30 minutes followed by solvent evaporation in vacuo. The crude product was dissolved in 2 mL DMF followed by addition of TCO-NHS (5.3 mg, 0.02 mmol) and TEA (10 mg, 0.1 mmol), the reaction was stirred at room temperature for 1 hour. After that the mixture was quenched by addition of 3 mL water, extracted with EtOAc (2×10 mL), concentrated and then purified by flash column (MeOH:DCM = 1:50) to give compound IB-3 (2.8 mg, 20% yield). 1H NMR (400 MHz, DMSO-d6) δ 8.25 (s, 1H), 7.69 – 7.62 (m, 2H), 7.48 – 7.40 (m, 2H), 7.22 – 7.09 (m, 5H), 6.65 (s, 1H), 6.54 (s, 1H), 5.57 (ddd, J = 14.9, 10.1, 4.1 Hz, 1H), 5.42 (t, J = 13.5 Hz, 1H), 4.69 (d, J = 16.8 Hz, 1H), 4.52 (d, J = 12.6 Hz, 1H), 4.25 – 4.00 (m, 3H), 3.74 (t, J = 11.3 Hz, 1H), 2.21 (d, J = 31.4 Hz, 4H), 2.11 (s, 1H), 2.01 – 1.78 (m, 6H), 1.58 (d, J = 24.2 Hz, 6H). HR-MS (m/z) [M + H]+ calcd: 705.3871; Found: 705.3866.
S1_5
4. Crystal structures of various kinase inhibitors with the corresponding main target proteins
(1)
(2)
- (4)
- (3)
(5)
Figure S1. Crystal structure view of (1) Afatinib/EGFR (4g5p), (2) Ibrutinib/BTK (5p9j), (3) JNK-IN-8/JNK3 (3v6s), (4)
XTF262/EGFRT790M (5gmp), (5) BLU9931/FGFR4 (4XCU), the amenable site of the parent inhibitors was red-colored.
5. In Vitro Enzymatic Activity Assay and Cell Growth Inhibition Assay[8]
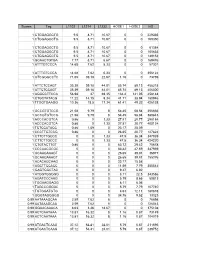
All the probes were evaluated with the EGFR, BTK kinase inhibition using Z'-LYTE™ fluorescence resonance energy transfer
(FRET) method, parent inhibitors were used as the reference compounds. The Z’-LYTE™ biochemical assay employs a FRET-based, coupled-enzyme format and is based on the differential sensitivity of phosphorylated and non-phosphorylated peptides to proteolytic cleavage. The peptide substrate is labeled with two fluorophores-one at each end-that make up a FRET pair. The compounds were diluted three-fold from 5.1x10-9 M to 1x10-4 M in DMSO. Plate was measured on EnVision Multilabel Reader (Perkin Elmer). Curve fitting and data presentations were performed using Graph Pad Prism version 4.0. Cytotoxicity assays were carried out using A431 and Raji cells by CCK8 assay. 3000 cells per well were seeded in a 96-well plate (100 μL medium/well) and incubated for 24 h in a humidified incubator for adherence. The probes and parent inhibitors in DMSO were added to cells at the final concentrations
(DMSO never exceeded 1‰) of 31.2, 15.6, 7.8, 3.9, 1.95, 0.97, 0.48, 0.24, 0.12, 0.06, 0.03 and 0.015 μM and further incubated for 48 h. CCK-8 reagent (10 μL) was added to each well and incubated for 2 h. Following that, the absorbance was measured at 450 nm
and 650 nm on a plate reader (Synergy HI, BioTek Instruments, Inc. Vermont, US). Cell viability rate was determined as VR = (A – A0)/(As – A0) × 100%, where A is the absorbance of the experimental group, As is the absorbance of the control group (DMSO was used as the control) and A0 is the absorbance of the blank group (no cells). IC50 values were calculated using GraphPad Prism.
S1_6
1 0 0
5 0
0
1 0 0
5 0
0
A fa tin ib (IC 5 0
- =
- 2 .5 4
- ±
- 0 .1 3 n M )
A fa tin ib (IC 5 0
- =
- 0 .3 7
- ±
- 0 .0 6 n M )
A F -1 (IC 5 0
==
8 .1 8 ± 1 .8 1 n M ) 5 .0 8 ± 0 .4 2 n M )
A F -1 (IC 5 0
==
0 .6 4 0 .6 3
±±
0 .1 8 n M ) 0 .0 6 n M
A F -2 (IC 5 0
A F -2 (IC 5 0
)
E G F R W T
E G F R T 7 9 0 M
- -1 0
- -9
- -8
- -7
- -6
- -1 0
- -9
- -8
- -7
- -6
- -5
- -4
L o g [In h ]/M
L o g [In h ]/M
A fa tin ib (IC 50
- =
- 2 .2 9
- ±
- 0 .3 μ M )
Ib ru tin ib (IC 50
- =
- 1.7
- ±
- 0.2 nM )
1 0 0
A F -1 (IC 50
==
5.02 7.79
- ±
- 0.1 μM )
0.1 μM
IB -1 (IC 50
- =
- 75.0
- ±
- 11.0 nM )
4.2 nM )
1 0 0
A F -2 (IC 50
- ±
- )
IB -2 (IC 50 =21.0
±
IB -3 (IC 50