Environmental Health & Safety
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
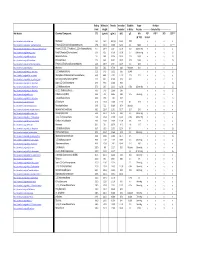
Detectable Compounds by MIP.Pdf
Boiling Molecular Density Ionization Solubility Vapor Analytes Point Weight Potential in Water Pressure ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐Detected By‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Wiki Website Chemical Compound: (oC) (g/mol) (g/mL) (eV) g/L kPa PID* FID** XSD ECD*** @ ~25C 10.6 eV https://en.wikipedia.org/wiki/Methane . Methane ‐161 16.0 .657 g/L 12.61 0.02 n y n n https://en.wikipedia.org/wiki/Dichlorodifluoromethane . Freon 12 (Dichlorodifluoromethane) ‐29.8 120.9 1.486 12.31 0.3 568.0 n y y LS**** https://en.wikipedia.org/wiki/1,1,2‐Trichloro‐1,2,2‐trifluoroethane . Freon 113 (1,1,2‐Trichloro‐1,2,2‐trifluoroethane) 47.7 187.4 1.564 11.78 0.17 285mm Hg n y y y https://en.wikipedia.org/wiki/Vinyl_chloride . Vinyl Chloride (Chloroethene) ‐13.0 62.5 0.910 10.00 2.7 2580mm Hg y y y LS https://en.wikipedia.org/wiki/Bromomethane . Bromomethane 3.6 94.9 3.974 10.53 17.5 190.0 n y y LS https://en.wikipedia.org/wiki/Chloroethane .. Chloroethane 12.3 64.5 0.901 10.97 5.74 134.6 n y y LS https://en.wikipedia.org/wiki/Trichlorofluoromethane . Freon 11 (Trichlorofluoromethane) 23.8 137.4 1.494 11.77 1.1 89.0 n y y y https://en.wikipedia.org/wiki/Acetone . Acetone 56.5 58.1 0.790 9.69 Miscible 30.6 y y n n https://en.wikipedia.org/wiki/1,1‐Dichloroethene . 1,1‐Dichloroethene 32.0 97.0 1.213 9.65 0.04% y y y LS https://en.wikipedia.org/wiki/Dichloromethane . -

Exposure Investigation Protocol: the Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH
Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan Exposure Investigation Protocol: The Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH MDCH/ATSDR - 2004 Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan TABLE OF CONTENTS OBJECTIVE/PURPOSE..................................................................................................... 3 RATIONALE...................................................................................................................... 4 BACKGROUND ................................................................................................................ 5 AGENCY ROLES .............................................................................................................. 6 ESTABLISHING CRITERIA ............................................................................................ 7 “Odor Events”................................................................................................................. 7 Comparison Values......................................................................................................... 7 METHODS ....................................................................................................................... 12 Instantaneous (“Grab”) Air Sampling........................................................................... 12 Continuous Air Monitoring.......................................................................................... -

Product Stewardship Summary Carbon Tetrachloride
Product Stewardship Summary Carbon Tetrachloride May 31, 2017 version Summary This Product Stewardship Summary is intended to give general information about Carbon Tetrachloride. It is not intended to provide an in-depth discussion of all health and safety information about the product or to replace any required regulatory communications. Carbon tetrachloride is a colorless liquid with a sweet smell that can be detected at low levels; however, odor is not a reliable indicator that occupational exposure levels have not been exceeded. Its chemical formula is CC14. Most emissive uses of carbon tetrachloride, a “Class I” ozone-depleting substance (ODS), have been phased out under the Montreal Protocol on Substances that Deplete the Ozone Layer (Montreal Protocol). Certain industrial uses (e.g. feedstock/intermediate, essential laboratory and analytical procedures, and process agent) are allowed under the Montreal Protocol. 1. Chemical Identity Name: carbon tetrachloride Synonyms: tetrachloromethane, methane tetrachloride, perchloromethane, benzinoform Chemical Abstracts Service (CAS) number: 56-23-5 Chemical Formula: CC14 Molecular Weight: 153.8 Carbon tetrachloride is a colorless liquid. It has a sweet odor, and is practically nonflammable at ambient temperatures. 2. Production Carbon tetrachloride can be manufactured by the chlorination of methyl chloride according to the following reaction: CH3C1+ 3C12 —> CC14 + 3HC1 Carbon tetrachloride can also be formed as a byproduct of the manufacture of chloromethanes or perchloroethylene. OxyChem manufactures carbon tetrachloride at facilities in Geismar, Louisiana and Wichita, Kansas. Page 1 of 7 3. Uses The use of carbon tetrachloride in consumer products is banned by the Consumer Product Safety Commission (CPSC), under the Federal Hazardous Substance Act (FHSA) 16 CFR 1500.17. -

The Search for Alternative Refrigerants -- a Molecular Approach
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Purdue E-Pubs Purdue University Purdue e-Pubs International Refrigeration and Air Conditioning School of Mechanical Engineering Conference 1988 The eS arch for Alternative Refrigerants -- A Molecular Approach M. O. McLinden National Bureau of Standards D. A. Didion National Bureau of Standards Follow this and additional works at: http://docs.lib.purdue.edu/iracc McLinden, M. O. and Didion, D. A., "The eS arch for Alternative Refrigerants -- A Molecular Approach" (1988). International Refrigeration and Air Conditioning Conference. Paper 69. http://docs.lib.purdue.edu/iracc/69 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. Complete proceedings may be acquired in print and on CD-ROM directly from the Ray W. Herrick Laboratories at https://engineering.purdue.edu/ Herrick/Events/orderlit.html THE SEARCH FOR ALTERNATIVE REFRIGERANTS- -A MOLECULAR APPROACH Mark 0. McLinden and David A. Didion National Bureau of Standards Gaithersburg, MD 20899 ABSTRACT The impending prodllction limitations on the fully halogenated CFG refrigerants is causing an intense search for possible substitutes. This study reviews the historical development of the CFG refrigerants and the relationship between molecular structure and t:he ozone depletio11 and greenhouse warming potentials. It then demonstrates that the halogenated hydrocarbons (a class of compounds -

Toxicological Profile for Carbon Tetrachloride
CARBON TETRACHLORIDE 175 5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL 5.1 PRODUCTION Carbon tetrachloride is produced by exhaustive chlorination of a variety of low molecular weight hydrocarbons such as carbon disulfide, methane, ethane, propane, and ethylene dichloride (HSDB 2004). It is also produced by thermal chlorination of methyl chloride (HSDB 2004). Carbon tetrachloride is a feedstock for chlorofluorocarbon gases, such as dichlorodifluoromethane (F-12) and trichlorofluoro methane (F-11), which were used as aerosol propellants in the 1950s and 1960s (Holbrook 1991). Following this, the growth rate for the production of carbon tetrachloride averaged 10.7% per year from 1960 to 1970 (Holbrook 1991). This rate slowed to 7.2% per year from 1970 to 1974, when the production of this chemical was at its peak, as other forms of propellants became commercially available (Anonymous 1981; Holbrook 1991). The FDA banned the sale of carbon tetrachloride in any product used in the home and the EPA regulated the use of chlorofluorocarbon gases as aerosols or propellants. Since then, production of carbon tetrachloride has declined at approximately 8% a year from 1974 to 1994 (Anonymous 1995; Holbrook 1991). Carbon tetrachloride is currently manufactured in the United States by Vulcan Materials Company at two plants: Geismar, Louisiana and Wichita, Kansas, with a combined 130 million pound capacity (HSDB 2004; SRI 2004). It should be noted, however, that these capacities are flexible, since other chlorinated solvents are made using the same equipment (SRI 2004). This recent decline in production is due to the adoption of an international agreement (the Montreal Protocol) to reduce environmental concentrations of ozone-depleting chemicals (including carbon tetrachloride), and to the provisions of Title VI of the Clean Air Act Amendments of 1990 addressing these chemicals. -

SROC Annex V
Annex V Major Chemical Formulae and Nomenclature This annex presents the formulae and nomenclature for halogen-containing species and other species that are referred to in this report (Annex V.1). The nomenclature for refrigerants and refrigerant blends is given in Annex V.2. V.1 Substances by Groupings V.1.1 Halogen-Containing Species V.1.1.1 Inorganic Halogen-Containing Species Atomic chlorine Cl Atomic bromine Br Molecular chlorine Cl2 Molecular bromine Br2 Chlorine monoxide ClO Bromine monoxide BrO Chlorine radicals ClOx Bromine radicals BrOx Chloroperoxy radical ClOO Bromine nitrate BrONO2, BrNO3 Dichlorine peroxide (ClO dimer) (ClO)2, Cl2O2 Potassium bromide KBr Hydrogen chloride (Hydrochloric acid) HCl Inorganic chlorine Cly Antimony pentachloride SbCl5 Atomic fluorine F Molecular fluorine F2 Atomic iodine I Hydrogen fluoride (Hydrofluoric acid) HF Molecular iodine I2 Sulphur hexafluoride SF6 Nitrogen trifluoride NF3 IPCC Boek (dik).indb 467 15-08-2005 10:57:13 468 IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System V.1.1.2 Halocarbons For each halocarbon the following information is given in columns: • Chemical compound [Number of isomers]1 (or common name) • Chemical formula • CAS number2 • Chemical name (or alternative name) V.1.1.2.1 Chlorofluorocarbons (CFCs) CFC-11 CCl3F 75-69-4 Trichlorofluoromethane CFC-12 CCl2F2 75-71-8 Dichlorodifluoromethane CFC-13 CClF3 75-72-9 Chlorotrifluoromethane CFC-113 [2] C2Cl3F3 Trichlorotrifluoroethane CCl FCClF 76-13-1 CFC-113 2 2 1,1,2-Trichloro-1,2,2-trifluoroethane -

Carbon Tetrachloride
frodeFebruary 2017 Office of Chemical Safety and Pollution Prevention U.S. EPA Preliminary Information on Manufacturing, Processing, Distribution, Use, and Disposal: Carbon Tetrachloride CASRN: 56-23-5 February 2017 Support document for Docket EPA-HQ-OPPT-2016-0733 Page 1 of 21 This document provides a preliminary public summary of available information collected by EPA’s Office of Pollution Prevention and Toxics (OPPT) in the Office of Chemical Safety and Pollution Prevention (OCSPP) on the manufacturing (including importing), processing, distribution in commerce, use, and disposal of this chemical. This is based on existing data available to EPA, including information collected under the Chemical Data Reporting rule, Toxics Release Inventory, information from other Agency databases, other U.S. Government agencies, publicly available information from states, and a review of published literature. In addition, the document includes information reported to EPA by producers and users of the chemical in the United States and in other countries. This preliminary use information and any additional use information received in the docket by March 15, 2017 will inform efforts to develop the scope of the chemical risk evaluation required under section 6(b)(4) of the Toxic Substances Control Act, and will inform any risk management efforts following risk evaluation. Mention of trade names in this document does not constitute endorsement by EPA. To verify products or articles containing this chemical currently in commerce, EPA has identified several examples. Any lists are provided for informational purposes only. EPA and its employees do not endorse any of the products or companies. This document does not contain confidential business information (CBI). -

3017.0001 the Fluorides of the Actinide Elements
ADVANCES IN FLUORINE CHEMISTRY VOLUME 2 EDITOR8 M. STACEY, F.R.S. A,Iaso~t Professor and tIead qf Deparlment of Chemistry, University of Birmingham J. C. TATLOW, Ph.D., D.Sc. Professor of Organic Chemistry, University of Birmingham A. G. SHARPE, M.A., Ph.D. Universi!~ Lecturer in Chemisto,, Cambridge WASHINGTON BUTTERWORTHS 1961 3017.0001 THE FLUORIDES OF THE ACTINIDE ELEMENTS Templeton, D. H. and Dauben, C. H. J. Amer. Chem. Soc. 1953, 75, 4560 Carniglia, S. C. and Cunningham, B. B. J. Amer. Chem. Soc. 1955, 77, 1451 Westrum, E. F. and Eyring, L. J. Amer. Chem. Soc. 1951, 73, 3396 Feay, D. C. Some Chemical Properties of Curium, Thesis, University of California. See also U.S.A.E.C. Report, UCRL-2547, 1954 Eyring, L., Cunningham, B. B. and Lohr, H. R. J. Amer. CAem. Soc. 1952, 74, 1186 Yakovlev, G. N. and Kosyakov, V. N. Proc. 2nd U.N. Co~fc. Pc’a@d Uses Atomic Energy, Geneva, 1958, paper 2127, 28, 373, 1958 Asprey, L. B. and Keenan, T. K. J. Inorg. Nuclear Chem. 1958, 7, 27 Asprey, L. B., Ellinger, F. H. and Zacl~ariasen, W. H. J. Amer. Chem. Soc. 1954, 76, 5235 Wallmann, J. C., Crane, W. W. T. and Cunningttam, B. 13. J. Amer. Chem. Soc. 1951, "/3, 493 Asprey, L. B., Ellinger, F. H., Fried, S. and Zachariasen, W. H. J. Ame~’. CheDz. Soc. 1957, ~, 5825 Asprey, L. B. and Ellinger, F. H. U.S.A.E.C. Report, AECD-3627, declassified 1954 Crane, W. W. T. U.S.A.E.C. -

Classification of Refrigerants
INSTITUT INTERNATIONAL INTERNATIONAL INSTITUTE DU FROID OF REFRIGERATION Organisation intergouvernement ale Intergovernmental organization pour le développement du froid for the development of refrigeration 177, boulevard Malesherbes, 75017 PARIS, France - Tél. 33-(0)1 42 27 32 35 - Fax 33-(0)1 47 63 17 98 - E-mail : [email protected] - Web : www.iifiir.org Classification of refrigerants This text is based on the American standard ANSI/ASHRAE 34 published in 2001 and entitled “Designation and Safety Classification of Refrigerants”. This classification makes it possible to designate all refrigerants used in a clear and internationally recognized manner by classifying them according to their chemical composition. 1 Numbering of Refrigerants An identifying number shall be assigned to each refrigerant. It consists of a prefix made up of letters and a suffix made up of digits. 1.1 Prefixes The prefix is composed of the letter R (for refrigerant). Examples: R22, R134a, R600a, R717 Sometimes, the letter C is used in the prefix to denote carbon, preceded by B, C or F (or a combination of these letters in the same order) to indicate the presence of bromine, chlorine or fluorine. Compounds containing hydrogen must be preceded by the letter H. Examples: HCFC22, HFC134a These prefixes must only be used in non-technical publications. Note: the name of the brand or of the manufacturer is also used sometimes; these names must not be used in official documents (identification labels, etc.). 1.2 Suffixes • Hydrocarbons and derivatives The first digit on the right (units) is the number of fluorine (F) atoms. The second digit on the right (tens) is one more than the number of hydrogen (H) atoms. -

MATERIAL SAFETY DATA SHEET Prepared to U.S
MATERIAL SAFETY DATA SHEET Prepared to U.S. OSHA, CMA, ANSI, Canadian WHMIS, Australian WorkSafe, Japanese Industrial Standard JIS Z 7250:2000, and European Union REACH Regulations SECTION 1 - PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: NON-FLAMMABLE GAS MIXTURE Containing One of the Following Components in a Nitrogen or Air Balance Gas: Dichlorodifluoromethane, 0.0005-2.0%;Trichlorofluoromethane, 0.0005-2.0%;1, 1, 2- Trichloro-1, 1, 2- trifluoroethane, 0.0005-2.0%; Tetrafluoroethane, 0.0005- 2.0% SYNONYMS: Not Applicable CHEMICAL FAMILY NAME: Not Applicable FORMULA: Not Applicable PRODUCT USE: Calibration of Monitoring and Research Equipment DOCUMENT NUMBER: MSDS 1088 (99-0263, 99-0281) U.N. NUMBER: UN 1956 U.N. DANGEROUS GOODS CLASS: 2.2 (Non-Flammable(Non Flammable Gas)G SUPPLIER/MANUFACTURER'S NAME: PortaGAS, Inc. ADDRESS: 1202 E. Sam Houston Pkwy S., Pasadena, TX 77503 EMERGENCY PHONE: TOLL-FREE in USA/Canada: (800)255-3924 International calls: +1 813 248 0585 Australian Poison Control: 13 11 26 Australian Fire Brigade: 000 BUSINESS PHONE: (713) 928-6477 General MSDS Info DATE OF PREPARATION: Oct 2012 DATE OF LAST REVISION: Oct 2012 SECTION 2 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW: This gas mixture is a colorless, odorless gas. Releases of this gas mixture for which Nitrogen is the balance gas may produce oxygen-deficient atmospheres (especially in confined spaces or other poorly-ventilated environments); individuals in such atmospheres may be asphyxiated. Components of this gas mixture (1,1,2-Trichloro-1,1,2- trifluoroethane, Tetrafluoromethane, Trichlorofluoromethane, and Dichlorodifluoromethane) may cause drowsiness and other central nervous system effects in high concentrations; however, due to their low concentration in this gas mixture, this is unlikely to occur. -

Pollution Characteristics and Health Risk Assessment of Summertime Atmospheric Volatile Halogenated Hydrocarbons in a Typical Urban Area of Beijing, China
atmosphere Article Pollution Characteristics and Health Risk Assessment of Summertime Atmospheric Volatile Halogenated Hydrocarbons in a Typical Urban Area of Beijing, China 1,2, 1,3, 1, 1 4 1,5 Yuanyuan Ji y, Linghong Xu y, Hong Li *, Chuhan Wang , Dongyao Xu , Lei Li , Hao Zhang 1, Jingchun Duan 1, Yujie Zhang 1, Xuezhong Wang 1, Weiqi Zhang 1 , Fang Bi 1, Yizhen Chen 1, Yanting Yu 6 and Lingshuo Meng 1 1 State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China; [email protected] (Y.J.); [email protected] (L.X.); [email protected] (C.W.); [email protected] (L.L.); [email protected] (H.Z.); [email protected] (J.D.); [email protected] (Y.Z.); [email protected] (X.W.); [email protected] (W.Z.); [email protected] (F.B.); [email protected] (Y.C.); [email protected] (L.M.) 2 College of Earth Sciences, Jilin University, Changchun 130061, China 3 Daishan Environmental Protection Bureau, Zhoushan 316200, China 4 School of Chemical & Environmental Engineering, China University of Mining and Technology, Beijing 100083, China; [email protected] 5 Academy of Environmental Planning and Design, Co., Ltd., Nanjing University, Nanjing 210093, China 6 Puyang Research Institute of Environmental Sciences, Puyang 457000, China; [email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 16 August 2020; Accepted: 16 September 2020; Published: 23 September 2020 Abstract: Twenty-three atmospheric volatile halogenated hydrocarbons (VHHs) were detected in a typical urban area of Beijing, China from 24 August to 4 September, 2012. -

Epa Hazardous Waste Codes
EPA HAZARDOUS WASTE CODES Code Waste description Code Waste description D001 Ignitable waste D023 o-Cresol D002 Corrosive waste D024 m-Cresol D003 Reactive waste D025 p-Cresol D004 Arsenic D026 Cresol D005 Barium D027 1,4-Dichlorobenzene D006 Cadmium D028 1,2-Dichloroethane D007 Chromium D029 1,1-Dichloroethylene D008 Lead D030 2,4-Dinitrotoluene D009 Mercury D031 Heptachlor (and its epoxide) D010 Selenium D032 Hexachlorobenzene D011 Silver D033 Hexachlorobutadiene D012 Endrin(1,2,3,4,10,10-hexachloro-1,7-epoxy- D034 Hexachloroethane 1,4,4a,5,6,7,8,8a-octahydro-1,4-endo, endo- 5,8-dimeth-ano-naphthalene) D035 Methyl ethyl ketone D013 Lindane (1,2,3,4,5,6-hexa- D036 Nitrobenzene chlorocyclohexane, gamma isomer) D037 Pentachlorophenol D014 Methoxychlor (1,1,1-trichloro-2,2-bis [p- methoxyphenyl] ethane) D038 Pyridine D015 Toxaphene (C10 H10 Cl8, Technical D039 Tetrachloroethylene chlorinated camphene, 67-69 percent chlorine) D040 Trichlorethylene D016 2,4-D (2,4-Dichlorophenoxyacetic acid) D041 2,4,5-Trichlorophenol D017 2,4,5-TP Silvex (2,4,5- D042 2,4,6-Trichlorophenol Trichlorophenoxypropionic acid) D043 Vinyl chloride D018 Benzene D019 Carbon tetrachloride D020 Chlordane D021 Chlorobenzene D022 Chloroform B-1 EPA HAZARDOUS WASTE CODES (Continued) Code Waste description Code Waste description HAZARDOUS WASTE FROM NONSPECIFIC solvents: cresols, cresylic acid, and SOURCES nitrobenzene; and the still bottoms from the recovery of these solvents; all spent solvent F001 The following spent halogenated solvents mixtures/blends containing, before use, a used in degreasing: Tetrachloroethylene, total of ten percent or more (by volume) of trichlorethylene, methylene chloride, 1,1,1- one or more of the above nonhalogenated trichloroethane, carbon tetrachloride and solvents or those solvents listed in F001, chlorinated fluorocarbons; all spent solvent F002, and F005; and still bottoms from the mixtures/blends used in degreasing recovery of these spent solvents and spent containing, before use, a total of ten solvent mixtures.