3017.0001 the Fluorides of the Actinide Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regulated Substance List
INSTRUCTIONS FOR THE UNIFIED PROGRAM (UP) FORM REGULATED SUBSTANCE LIST CHEMICAL NAME CAS # TQ Listing CHEMICAL NAME CAS # TQ Listing (Lbs) Basis (Lbs) Basis Acetaldehyde 75-07-0 10,000 g Cantharidin 56-25-7 100/10,0001 * Acetone Cyanohydrin 75-86-5 1,000 Carbachol Chloride 51-83-2 500/10,0001 Acetone Thiosemicarbazide 1752-30-3 1,000/10,0001 Acetylene (Ethyne) 74-86-2 10,000 f Carbamic Acid, Methyl-,o- Acrolein (2-Propenal) 107-02-8 500 b (((2,4-Dimethyl-1,3-Dithiolan- Acrylamide 79-06-1 1,000/10,0001 2-YL) Methylene)Amino)- 26419-73-8 100/10,0001 Acrylonitrile (2- Propenenitrile) 107-13-1 10,000 b Carbofuran 1563-66-2 10/10,0001 Acrylyl Chloride Carbon Disulfide 75-15-0 10,000 b (2-Propenoyl Chloride) 814-68-6 100 b Carbon Oxysulfide Aldicarb 116-06-3 100/10,0001 (Carbon Oxide Sulfide (COS)) 463-58-1 10,000 f Aldrin 309-00-2 500/10,0001 Chlorine 7782-50-5 100 a,b Allyl Alcohol (2-Propen-1-ol) 107-18-6 1,000 b Chlorine Dioxide Allylamine (2-Propen-1-Amine) 107-11-9 500 b (Chlorine Oxide (ClO2)) 10049-04-4 1,000 c Aluminum Phosphide 20859-73-8 500 Chlorine Monoxide (Chlorine Oxide) 7791-21-1 10,000 f Aminopterin 54-62-6 500/10,0001 Chlormequat Chloride 999-81-5 100/10,0001 Amiton Oxalate 3734-97-2 100/10,0001 Chloroacetic Acid 79-11-8 100/10,0001 Ammonia, Anhydrous 2 7664-41-7 500 a,b Chloroform 67-66-3 10,000 b Ammonia, Aqueous Chloromethyl Ether (conc 20% or greater) 7664-41-7 20,000 a,b (Methane,Oxybis(chloro-) 542-88-1 100 b * Aniline 62-53-3 1,000 Chloromethyl Methyl Ether Antimycin A 1397-94-0 1,000/10,0001 (Chloromethoxymethane) -

EPCRA.Doc Elizabethtown College
Environmental Affairs EMERGENCY PLANNING AND COMMUNITY RIGHT TO KNOW SCOPE: This policy includes all hazardous materials that are stored and/or used on site and for which there is an MSDS required by OSHA 1910:1200 Hazard Communication. PURPOSE: The purpose of this policy is to establish reporting requirements to provide the public with information on the hazardous chemicals used and or stored at the College. It also is intended to facilitate the development of state and local emergency response plans. PROCESS: ♦ The local emergency planning committee has been notified that the Manager of Human Resources & Safety will serve as the Facility Emergency Coordinator. ♦ The College will provide the Local Emergency Planning Committee and the State Emergency Response Commission with all information needed for emergency planning. ♦ The College will collect and maintain copies of all MSDS(s) required under OSHA 1910:1200 Hazard Communication. The College also subscribes to an MSDS On Demand service so that MSDS(s) will be available at all times. ♦ The College has provided the State Emergency Response Commission, the Local Emergency Planning Committee and the local Fire Department with a complete list of hazardous chemicals used/stored at the College in preference to complete sets of MSDS(s). The College will notify the above agencies of significant changes in the inventory levels of extremely hazardous substances (EHS). ♦ As a matter of practice the College will maintain inventory levels of EHS(s) campus-wide below the Threshold Planning Quantities (TPQ) or 500 lbs, whichever is less, whenever possible. The exception to this will be Sulfuric Acid because of its presence in batteries. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -
Detectable Compounds by MIP.Pdf
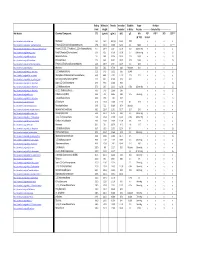
Boiling Molecular Density Ionization Solubility Vapor Analytes Point Weight Potential in Water Pressure ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐Detected By‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Wiki Website Chemical Compound: (oC) (g/mol) (g/mL) (eV) g/L kPa PID* FID** XSD ECD*** @ ~25C 10.6 eV https://en.wikipedia.org/wiki/Methane . Methane ‐161 16.0 .657 g/L 12.61 0.02 n y n n https://en.wikipedia.org/wiki/Dichlorodifluoromethane . Freon 12 (Dichlorodifluoromethane) ‐29.8 120.9 1.486 12.31 0.3 568.0 n y y LS**** https://en.wikipedia.org/wiki/1,1,2‐Trichloro‐1,2,2‐trifluoroethane . Freon 113 (1,1,2‐Trichloro‐1,2,2‐trifluoroethane) 47.7 187.4 1.564 11.78 0.17 285mm Hg n y y y https://en.wikipedia.org/wiki/Vinyl_chloride . Vinyl Chloride (Chloroethene) ‐13.0 62.5 0.910 10.00 2.7 2580mm Hg y y y LS https://en.wikipedia.org/wiki/Bromomethane . Bromomethane 3.6 94.9 3.974 10.53 17.5 190.0 n y y LS https://en.wikipedia.org/wiki/Chloroethane .. Chloroethane 12.3 64.5 0.901 10.97 5.74 134.6 n y y LS https://en.wikipedia.org/wiki/Trichlorofluoromethane . Freon 11 (Trichlorofluoromethane) 23.8 137.4 1.494 11.77 1.1 89.0 n y y y https://en.wikipedia.org/wiki/Acetone . Acetone 56.5 58.1 0.790 9.69 Miscible 30.6 y y n n https://en.wikipedia.org/wiki/1,1‐Dichloroethene . 1,1‐Dichloroethene 32.0 97.0 1.213 9.65 0.04% y y y LS https://en.wikipedia.org/wiki/Dichloromethane . -

1080 Sodium Fluoroacetate
Wild dog facts 1080-Sodium fluoroacetate 1080 (sodium fluoroacetate) is used to control wild The owner must ensure that signs are put up dogs, feral pigs, foxes, cats and rabbits in immediately before any 1080 baits are put out Queensland. It occurs naturally in a number of on the property. native plant species including Acacia georginae The signs must be placed at all entrances to (Georgina gidgee) and members of the the property and at the extremities of property Gastrolobium and Oxylobium genera. Sodium boundaries that front a public thoroughfare. fluoroacetate is a fluffy white material at room This must be done even if the adjoining temperature, which forms colourless solutions property is carrying out 1080 baiting. with water and is normally odourless. Signs must remain in place for a period of 4 weeks after baits are laid and permanent How to access 1080 signs are required for on-going or extended baiting program. Only authorised persons can supply 1080 baits to landholders.The use of 1080 is subject to strict Toxicity of 1080 regulatory control according to which: Dogs and foxes are highly susceptible to Minimum distances 1080, and the small amount required to No baits are to be laid within 5 m of a fenced target these species poses a minimal threat boundary. to non-target species. Feral pigs and rabbits No baits are to be laid within 50 m of the are also susceptible, although higher doses centre line of a declared road. are required. Table 1 compares the No baits are to be laid within 20 m of susceptibility of different animals to 1080. -

Downloads/DL Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGI SCHE BEWERTUNGEN/Bewertungen/Toxbew072-L.Pdf
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: _____________________________ ______________ Jedidiah Samuel Snyder Date Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Master of Public Health Global Environmental Health _________________________________________ P. Barry Ryan, Ph.D. Committee Chair _________________________________________ Eugene Demchuk, Ph.D. Committee Member _________________________________________ Paige Tolbert, Ph.D. Committee Member Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Bachelor of Science in Engineering, B.S.E. The University of Iowa 2010 Thesis Committee Chair: P. Barry Ryan, Ph.D. An abstract of A thesis submitted to the Faculty of the Rollins School of Public Health of Emory University in partial fulfillment of the requirements for the degree of Master of Public Health in Global Environmental Health 2015 Abstract Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. -

Calarp) Program
California Accidental Release Prevention (CalARP) Program Administering Agency Guidance January 31, 2005 Preface This document provides general guidance to help Administering Agencies (AAs) implement and enforce the California Accidental Release Prevention (CalARP) Program. The intent is to identify the elements of the Program applicable to each regulated business, and assist AAs with oversight of the CalARP Program statutes and regulations. This document is not a substitute for the CalARP Program regulations; it does not impose legally binding requirements. About This Document This document follows the format of the California Code of Regulations, Title 19, Division 2, Chapter 4.5: California Accidental Release Prevention (CalARP) Program. The regulatory sections are presented in parentheses for ease of reference. Acknowledgements The California Emergency Management Agency (Cal EMA) would like to thank the following people for their valuable assistance in the preparation of this document: Howard Wines, Hazardous Materials Specialist, City of Bakersfield Fire Department Robert Distaso P.E., Fire Safety Engineer, Orange County Fire Authority Randall L. Sawyer, Supervisor, Accidental Release Prevention Programs, Contra Costa County Health Services Department Beronia Beniamine, Senior Hazardous Materials Specialist, Stanislaus County Environmental Resources Department Angie Proboszcz, Risk Management Program Coordinator, USEPA Region 9 Jon Christenson, Senior Environmental Health Specialist, Merced County Department of Public Health Teresa -

Exposure Investigation Protocol: the Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH
Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan Exposure Investigation Protocol: The Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH MDCH/ATSDR - 2004 Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan TABLE OF CONTENTS OBJECTIVE/PURPOSE..................................................................................................... 3 RATIONALE...................................................................................................................... 4 BACKGROUND ................................................................................................................ 5 AGENCY ROLES .............................................................................................................. 6 ESTABLISHING CRITERIA ............................................................................................ 7 “Odor Events”................................................................................................................. 7 Comparison Values......................................................................................................... 7 METHODS ....................................................................................................................... 12 Instantaneous (“Grab”) Air Sampling........................................................................... 12 Continuous Air Monitoring.......................................................................................... -

Elemental Fluorine Product Information (Pdf)
Elemental Fluorine Contents 1 Introduction ............................................................................................................... 4 2.1 Technical Application of Fluorine ............................................................................. 5 2.2 Electronic Application of Fluorine ........................................................................... 7 2.3 Fluorine On-Site Plant ............................................................................................ 8 3 Specifications ............................................................................................................ 9 4 Safety ...................................................................................................................... 10 4.1 Maintenance of the F2 system .............................................................................. 12 4.2 First Aid ................................................................................................................ 13 5.1 Chemical Properties ............................................................................................. 14 5.2 Physical Data ....................................................................................................... 15 6 Toxicity .................................................................................................................... 18 7 Shipping and Transport ........................................................................................... 20 8 Environment ........................................................................................................... -

Federal Law and Vertebrate Pest Control
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Proceedings of the 1st Vertebrate Pest Vertebrate Pest Conference Proceedings Conference (1962) collection February 1962 FEDERAL LAW AND VERTEBRATE PEST CONTROL Justus C. Ward Director, Pesticides Regulation Division, Agricultural Research Service, U.S. Department of Agriculture Follow this and additional works at: https://digitalcommons.unl.edu/vpcone Part of the Environmental Health and Protection Commons Ward, Justus C., "FEDERAL LAW AND VERTEBRATE PEST CONTROL" (1962). Proceedings of the 1st Vertebrate Pest Conference (1962). 25. https://digitalcommons.unl.edu/vpcone/25 This Article is brought to you for free and open access by the Vertebrate Pest Conference Proceedings collection at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Proceedings of the 1st Vertebrate Pest Conference (1962) by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. FEDERAL LAW AND VERTEBRATE PEST CONTROL By: Justus C. Ward, Director, Pesticides Regulation Division, Agricultural Research Service, U.S. Department of Agriculture Presented at the Vertebrate Pest Control Conference, Sacramento, California, February 6 and 7, 1 962 Shortly after the passage of the Federal Insecticide Act of 1910> mammal control specialists in the Bureau of Biological Survey began to consider a similar law to cover the chemicals with which they were concerned. Work on the project went slowly a nd spasmodically, but reached the point of having a Federal Rodenticide Act available for study and possible revision in 1928. At this time, the mammal control chemicals in use were limited to strychnine -- alkaloid and sulphate -arsenic, barium carbonate, th allium sulphate, phosphorus, s odium and calcium cyanide, carbon disulphide, and red squill. -

Hexafluoroethane
Common Name: HEXAFLUOROETHANE CAS Number: 76-16-4 RTK Substance number: 0989 DOT Number: UN 2193 Date: May 1986 Revision: March 1999 ----------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY * Hexafluoroethane can affect you when breathed in. * If you think you are experiencing any work-related health * Contact can cause severe skin and eye irritation and burns problems, see a doctor trained to recognize occupational with possible eye damage. diseases. Take this Fact Sheet with you. * Breathing Hexafluoroethane can irritate the nose and throat. WORKPLACE EXPOSURE LIMITS * Breathing Hexafluoroethane can irritate the lungs causing No occupational exposure limits have been established for coughing and/or shortness of breath. Higher exposures Hexafluoroethane. This does not mean that this substance is can cause a build-up of fluid in the lungs (pulmonary not harmful. Safe work practices should always be followed. edema), a medical emergency, with severe shortness of breath. * Large amounts of Hexafluoroethane will decrease the * Exposure to the liquified gas can cause frostbite. amount of available Oxygen. Oxygen content should be * Hexafluoroethane can cause headache, nausea, fatigue, routinely tested to ensure that it is at least 19% by volume. mood changes, dizziness, unconsciousness and death. * Very high exposure can cause suffocation from lack of WAYS OF REDUCING EXPOSURE Oxygen. It may also cause the heart to beat irregularly * Where possible, enclose operations and use local exhaust (arrhythmia) or to stop, which can be fatal. ventilation at the site of chemical release. If local exhaust ventilation or enclosure is not used, respirators should be IDENTIFICATION worn. Hexafluoroethane is a colorless and odorless gas, or liquid * Wear protective work clothing. -

The List of Extremely Hazardous Substances)
APPENDIX A (THE LIST OF EXTREMELY HAZARDOUS SUBSTANCES) THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 75-86-5 ACETONE CYANOHYDRIN 500 10 1752-30-3 ACETONE THIOSEMICARBAZIDE 500/500 1,000 107-02-8 ACROLEIN 500 1 79-06-1 ACRYLAMIDE 500/500 5,000 107-13-1 ACRYLONITRILE 500 100 814-68-6 ACRYLYL CHLORIDE 100 100 111-69-3 ADIPONITRILE 500 1,000 116-06-3 ALDICARB 100/500 1 309-00-2 ALDRIN 500/500 1 107-18-6 ALLYL ALCOHOL 500 100 107-11-9 ALLYLAMINE 500 500 20859-73-8 ALUMINUM PHOSPHIDE 500 100 54-62-6 AMINOPTERIN 500/500 500 78-53-5 AMITON 500 500 3734-97-2 AMITON OXALATE 100/500 100 7664-41-7 AMMONIA 500 100 300-62-9 AMPHETAMINE 500 1,000 62-53-3 ANILINE 500 5,000 88-05-1 ANILINE,2,4,6-TRIMETHYL- 500 500 7783-70-2 ANTIMONY PENTAFLUORIDE 500 500 1397-94-0 ANTIMYCIN A 500/500 1,000 86-88-4 ANTU 500/500 100 1303-28-2 ARSENIC PENTOXIDE 100/500 1 THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 1327-53-3 ARSENOUS OXIDE 100/500 1 7784-34-1 ARSENOUS TRICHLORIDE 500 1 7784-42-1 ARSINE 100 100 2642-71-9 AZINPHOS-ETHYL 100/500 100 86-50-0 AZINPHOS-METHYL 10/500 1 98-87-3 BENZAL CHLORIDE 500 5,000 98-16-8 BENZENAMINE, 3-(TRIFLUOROMETHYL)- 500 500 100-14-1 BENZENE, 1-(CHLOROMETHYL)-4-NITRO- 500/500 500 98-05-5 BENZENEARSONIC ACID 10/500 10 3615-21-2 BENZIMIDAZOLE, 4,5-DICHLORO-2-(TRI- 500/500 500 FLUOROMETHYL)- 98-07-7 BENZOTRICHLORIDE 100 10 100-44-7 BENZYL CHLORIDE 500 100 140-29-4 BENZYL CYANIDE 500 500 15271-41-7 BICYCLO[2.2.1]HEPTANE-2-CARBONITRILE,5-