Carbon Tetrachloride (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toxicological Profile for Carbon Tetrachloride
CARBON TETRACHLORIDE 1 1. PUBLIC HEALTH STATEMENT This public health statement tells you about carbon tetrachloride and the effects of exposure to it. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites are then placed on the National Priorities List (NPL) and are targeted for long-term federal clean-up activities. Carbon tetrachloride has been found in at least 430 of the 1,662 current or former NPL sites. Although the total number of NPL sites evaluated for this substance is not known, the possibility exists that the number of sites at which carbon tetrachloride is found may increase in the future as more sites are evaluated. This information is important because these sites may be sources of exposure and exposure to this substance may harm you. When a substance is released either from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. Such a release does not always lead to exposure. You can be exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking the substance, or by skin contact. If you are exposed to carbon tetrachloride, many factors will determine whether you will be harmed. These factors include the dose (how much), the duration (how long), and how you come in contact with it. You must also consider any other chemicals you are exposed to and your age, sex, diet, family traits, lifestyle, and state of health. -

Chemistry Department 32.Pdf (10.36Mb)
THE FABRICATION OF NANOMETRIC METAL SULFIDES FROM XANTHATE PRECURSORS By ALAN PIQUETTE Bachelor of Arts Western State College of Colorado Gunnison, Colorado 2002 Submitted to the Faculty of the Graduate College of the Oklahoma State University In partial fulfillment of The requirements for The Degree of DOCTOR OF PHILOSOPHY December, 2007 THE FABRICATION OF NANOMETRIC METAL SULFIDES FROM XANTHATE PRECURSORS Thesis Approved: _______________ __Allen Apblett___ ______________ Thesis Advisor ________________ Nicholas Materer_____ __________ _____________ __LeGrande Slaughter___ __________ _______________ ____Jim Smay___ _______________ _____________ __A. Gordon Emslie___ ____________ Dean of the Graduate College ACKNOWLEDGEMENTS It is with my utmost sincere appreciation that I acknowledge my thesis advisor, Dr. Allen W. Apblett, for his support, guidance, and motivation. He provided a research environment that was both friendly and challenging, which made my years in his group enjoyable and rewarding. His broad range of knowledge was something of which I was happy to take advantage. His dedication to teaching and research is something that will be a positive influence on me for the rest of my scientific career. I would like to express gratitude to my committee members: Dr. Nicholas Materer, Dr. LeGrande Slaughter, and Dr. Jim Smay, for their assistance, advice, guidance, and support throughout the years. I am deeply grateful to all my colleagues and friends in the chemistry department. I would specifically like to thank Sulaiman Al-Fadul, Mohammed Al-Hazmi, Zeid Al- Othmann, Mohamed Chehbouni, Satish Kuriyavar, and Tarek Trad for showing me the ropes, for their valuable discussions, continuous encouragement, and for all the help they extended during the course of my stay in the Apblett Group. -

Hydrogen and Carbon Isotope Fractionation During Degradation Of
ORIGINAL RESEARCH Hydrogen and carbon isotope fractionation during degradation of chloromethane by methylotrophic bacteria Thierry Nadalig1, Markus Greule2, Francßoise Bringel1,Stephane Vuilleumier1 & Frank Keppler2 1Equipe Adaptations et Interactions Microbiennes dans l’Environnement, UMR 7156 Universite de Strasbourg - CNRS, 28 rue Goethe, Strasbourg, 67083, France 2Max Planck Institute for Chemistry, Hahn-Meitner-Weg 1, 55128 Mainz, Germany Keywords Abstract Carbon isotope fractionation, chloromethane biodegradation, hydrogen isotope Chloromethane (CH3Cl) is a widely studied volatile halocarbon involved in the fractionation, methylotrophic bacteria. destruction of ozone in the stratosphere. Nevertheless, its global budget still remains debated. Stable isotope analysis is a powerful tool to constrain fluxes Correspondence of chloromethane between various environmental compartments which involve Thierry Nadalig, Universite de Strasbourg, a multiplicity of sources and sinks, and both biotic and abiotic processes. In UMR 7156 UdS-CNRS, 28 rue Goethe, this study, we measured hydrogen and carbon isotope fractionation of the 67083 Strasbourg Cedex, France. Tel: +33 3 68851973; Fax: +33 3 68852028; remaining untransformed chloromethane following its degradation by methylo- Methylobacterium extorquens Hyphomicrobium E-mail: [email protected] trophic bacterial strains CM4 and sp. MC1, which belong to different genera but both use the cmu pathway, the Funding Information only pathway for bacterial degradation of chloromethane characterized so far. Financial support for the acquisition of Hydrogen isotope fractionation for degradation of chloromethane was deter- GC-FID equipment by REALISE (http://realise. mined for the first time, and yielded enrichment factors (e)ofÀ29& and unistra.fr), the Alsace network for research À27& for strains CM4 and MC1, respectively. In agreement with previous and engineering in environmental sciences, is studies, enrichment in 13C of untransformed CH Cl was also observed, and gratefully acknowledged. -
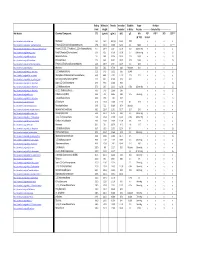
Detectable Compounds by MIP.Pdf
Boiling Molecular Density Ionization Solubility Vapor Analytes Point Weight Potential in Water Pressure ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐Detected By‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Wiki Website Chemical Compound: (oC) (g/mol) (g/mL) (eV) g/L kPa PID* FID** XSD ECD*** @ ~25C 10.6 eV https://en.wikipedia.org/wiki/Methane . Methane ‐161 16.0 .657 g/L 12.61 0.02 n y n n https://en.wikipedia.org/wiki/Dichlorodifluoromethane . Freon 12 (Dichlorodifluoromethane) ‐29.8 120.9 1.486 12.31 0.3 568.0 n y y LS**** https://en.wikipedia.org/wiki/1,1,2‐Trichloro‐1,2,2‐trifluoroethane . Freon 113 (1,1,2‐Trichloro‐1,2,2‐trifluoroethane) 47.7 187.4 1.564 11.78 0.17 285mm Hg n y y y https://en.wikipedia.org/wiki/Vinyl_chloride . Vinyl Chloride (Chloroethene) ‐13.0 62.5 0.910 10.00 2.7 2580mm Hg y y y LS https://en.wikipedia.org/wiki/Bromomethane . Bromomethane 3.6 94.9 3.974 10.53 17.5 190.0 n y y LS https://en.wikipedia.org/wiki/Chloroethane .. Chloroethane 12.3 64.5 0.901 10.97 5.74 134.6 n y y LS https://en.wikipedia.org/wiki/Trichlorofluoromethane . Freon 11 (Trichlorofluoromethane) 23.8 137.4 1.494 11.77 1.1 89.0 n y y y https://en.wikipedia.org/wiki/Acetone . Acetone 56.5 58.1 0.790 9.69 Miscible 30.6 y y n n https://en.wikipedia.org/wiki/1,1‐Dichloroethene . 1,1‐Dichloroethene 32.0 97.0 1.213 9.65 0.04% y y y LS https://en.wikipedia.org/wiki/Dichloromethane . -

United States Patent (113,607,865
United States Patent (113,607,865 (72) Inventors John Dyer (56) References Cited Media; UNITED STATES PATENTS Lyle H. Phifer, West Chester, both of Pa. 2,694,723 l l 11954 Schramm ..................... 260/455 B 2l Appl. No. 732,551 2,761,247 9/1956 Meadows.... a 260/216 22 Filed May 28, 1968 2,825,655 3/1958 Meadows..................... 260/216 45) Patented Sept. 21, 1971 2,910,466 101959 Watt............................. 260/216 73) Assignee FMC Corporation Philadelphia, Pa. 3.141023,103,507 9/19637/1964 O'Boyle...Knoevenagel. 260/234 Primary Examiner-Lewis Gotts (54) PREPARATION OFXANTHATES Assistant Examiner-Johnnie R. Brown 10 Claims, No Drawings Attorneys-Thomas R. O'Malley, George F. Mueller and Robert G. Hoffmann 52) U.S.C........................................................ 260/234 R, 260/26, 260/455 B 51 int. Cl......................................................... C07c69132 ABSTRACT: A method of forming alcohol xanthates utilizing 50 Field of Search............................................ 260/455 B, a transXanthation reaction between an alcohol xanthate and 234,216 an alcohol, is disclosed herein. 3,607,865 PREPARATION OFXANTHATES erythritol Alcohol xanthic acids and their derivatives are known to be sorbital useful for a variety of applications including mineral flotation glucose agents, sulfidizing agents, rubber vulcanization accelerators, mannitol adhesives and as intermediates in the preparation of shaped 2-methoxyethanol articles, for example, regenerated cellulose fibers and films. 2-ethoxyethanol In general, alcohol xanthates have been derived by reacting 2-butoxyethanol carbon disulfide with simple and complex alcohols under al diethyleneglycol ethylether kaline conditions. Reactions of this type have been known for polyethylene glycols many years and may be seen, for example, in many prior U.S. -

An Improved Method for BTEX Extraction From
Analytical Methods Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/methods Page 1 of 6 Analytical Methods 1 2 Analytical Methods RSC Publishing 3 4 5 TECHNICAL NOTE 6 7 8 9 An improved method for BTEX extraction from 10 charcoal 11 Cite this: DOI: 10.1039/x0xx00000x 12 13 Raffaele Cucciniello a, Antonio Proto a,*, Federico Rossi a, Nadia 14 b c 15 Marchettini , Oriana Motta Received 00th January 2015, 16 Accepted 00th January 2015 17 Abstract In this paper we propose a simple procedure for the extraction of BTEX (benzene, 18 DOI: 10.1039/x0xx00000x toluene, ethylbenzene and xylenes) from activated charcoal. For this purpose synthetic samples 19 www.rsc.org/ were prepared in laboratory and real samples were collected in a polluted environment using 20 21 passive sampling. -

Exposure Investigation Protocol: the Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH
Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan Exposure Investigation Protocol: The Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH MDCH/ATSDR - 2004 Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan TABLE OF CONTENTS OBJECTIVE/PURPOSE..................................................................................................... 3 RATIONALE...................................................................................................................... 4 BACKGROUND ................................................................................................................ 5 AGENCY ROLES .............................................................................................................. 6 ESTABLISHING CRITERIA ............................................................................................ 7 “Odor Events”................................................................................................................. 7 Comparison Values......................................................................................................... 7 METHODS ....................................................................................................................... 12 Instantaneous (“Grab”) Air Sampling........................................................................... 12 Continuous Air Monitoring.......................................................................................... -

12E4968079.Pdf
Fluorocarbon compatibilized gold-silica nanocomposites for recyclable regioselective hydroamination of alkynes in a fluorous biphasic system Item Type Conference Paper Authors Merican, Zulkifli; Vu, Bao Khanh; Solovyeva, Vera; Rodionov, Valentin; Khe, Cheng Seong; Rajalingam, Sokkalingam; Vasant, Pandian Citation Merican Z, Vu BK, Solovyeva VA, Rodionov VO, Khe CS, et al. (2016) Fluorocarbon compatibilized gold-silica nanocomposites for recyclable regioselective hydroamination of alkynes in a fluorous biphasic system. Available: http:// dx.doi.org/10.1063/1.4968079. Eprint version Publisher's Version/PDF DOI 10.1063/1.4968079 Publisher AIP Publishing Rights This article may be downloaded for personal use only. Any other use requires prior permission of the author and AIP Publishing. The following article appeared in Merican, Z., Vu, B.K., Solovyeva, V.A., Rodionov, V.O., Khe, C.S., Rajalingam, S. and Vasant, P., 2016, November. Fluorocarbon compatibilized gold-silica nanocomposites for recyclable regioselective hydroamination of alkynes in a fluorous biphasic system. In 4TH INTERNATIONAL CONFERENCE ON FUNDAMENTAL AND APPLIED SCIENCES (ICFAS2016) (Vol. 1787, No. 1, p. 030014). AIP Publishing and may be found at http://aip.scitation.org/doi/abs/10.1063/1.4968079. Download date 27/09/2021 23:14:23 Link to Item http://hdl.handle.net/10754/622088 Fluorocarbon compatibilized gold-silica nanocomposites for recyclable regioselective hydroamination of alkynes in a fluorous biphasic system Zulkifli Merican, Bao Khanh Vu, Vera A. Solovyeva, Valentin O. Rodionov, Cheng Seong Khe, Sokkalingam Rajalingam, and Pandian Vasant Citation: 1787, 030014 (2016); doi: 10.1063/1.4968079 View online: http://dx.doi.org/10.1063/1.4968079 View Table of Contents: http://aip.scitation.org/toc/apc/1787/1 Published by the American Institute of Physics Fluorocarbon Compatibilized Gold-Silica Nanocomposites For Recyclable Regioselective Hydroamination of Alkynes In A Fluorous Biphasic System Zulkifli Merican1, a Bao Khanh Vu2, Vera A. -

Low-Surface-Energy Fluoromethacrylate Block Copolymers with Patternable Elements
Chem. Mater. 2000, 12, 33-40 33 Low-Surface-Energy Fluoromethacrylate Block Copolymers with Patternable Elements Shu Yang, Jianguo Wang,† Kenji Ogino,‡ Suresh Valiyaveettil,§ and Christopher K. Ober* Department of Materials Science and Engineering, Cornell University, Bard Hall, Ithaca, New York 14853-1501 Received April 26, 1999. Revised Manuscript Received October 19, 1999 A series of block polymers were synthesized from tetrahydropyranyl methacrylate (THPMA) and several fluorinated methacrylates. By changing both the length of the fluorinated side chain and the nature of its end group, the surface properties of these polymers were greatly influenced. The polymers with perfluoroheptyl groups showed the lowest surface tension, ∼7 mN/m, whereas those with perfluoropropyl groups showed surface tension around 12 mN/m. The surface tension changed dramatically when the perfluorinated side chain with a CF2H end group was capped, increasing to 18 mN/m for three -CF2- units. The relative volume ratios of different block copolymers did not affect the resulting surface energy. After thermal decomposition of labile THP- groups in these polymers, acid and acid anhydride groups were produced. Thermal cross-linking of these groups increased mechanical robustness and led to better surface stability and adhesion of the polymer to the substrate. Because these polymers were designed for their lithographic abilities, some aspects of their photoimaging properties are discussed. Introduction surfactants, or emulsifiers,2 some of which are soluble in supercritical carbon dioxide.3,4 A monolayer of these Precise control of the surface properties of polymeric materials, properly organized, will make a very low- materials by means of self-organization is a major energy surface. -

JTEFT-04-00146.Pdf
Journal of Textile Engineering & Fashion Technology Research Article Open Access Process intensification of fluorocarbon-free and fluorocarbon-based water repellent finishes on cotton knit fabrics Abstract Volume 4 Issue 3 - 2018 The capabilities of the fluorocarbon-free alkyl urethane based resin, was analyzed Kawser Parveen Chowdhury on cotton fabric. In this study, both single jersey and double jersey knit structured Department of Wet Process Engineering, Bangladesh University fabrics were taken to evaluate the performance of different water repellent finishes of Textiles, Bangladesh; on fabrics properties. The performance of the fluorocarbon-free alkyl urethane based resin and fluorocarbon based water repellent chemicals were evaluated and compared Correspondence: Kawser Parveen Chowdhury, Assistant at different formulations. The effectiveness of water repellency of the finished fabrics Professor, Department of Wet Process Engineering, Bangladesh were evaluated by AATCC 127 hydrostatic head test method and by ISO 4920:2012 University of Textiles, Address: 92, Shaheed Tajuddin Ahmed spray rating test method. To assess the performance of water repellent finished knit Avenue, Tejgaon, Dhaka-1208, Bangladesh, Tel +8801716167777, fabrics, GSM, bursting strength test, stiffness, color fastness to wash, color fastness to Email [email protected] sea water, color fastness to saliva, color fastness to rubbing, color fastness to light were done according to ISO and ASTM method. The results showed that the fluorocarbon- Received: May 25, 2018 | Published: June 06, 2018 free alkyl urethane based resin treated fabrics exhibited competitive result on water repellency, other physical and chemicals properties. The water repellent finish type and concentration were very important criteria to obtain good water repellency. -

PAPERS READ BEFORE the CHEMICAL SOCIETY. XXII1.-On
View Article Online / Journal Homepage / Table of Contents for this issue 773 PAPERS READ BEFORE THE CHEMICAL SOCIETY. XXII1.-On Tetrabromide of Carbon. No. II. By THOMASBOLAS and CHARLESE. GROVES. IN a former paper* we described several methods for the preparation of the hitherto unknown tetrabromide of carbon, and in the present communication we desire to lay before the Society the results of our more recent experiments. In addition to those methods of obtaining the carbon tetrabromide, which we have already published, the fol- lowing are of interest, either from a theoretical point of view, or as affording advantageous means for the preparation of that substance. Action of Bromine on Carbon Disulphide. Our former statement that? bromine had no action on carbon disul- phide requires some modification, as we find that when it is heated to 180" or 200" for several hundred hours with bromine free from both chlorine and iodine, and the contents of the tubes are neutralised and distilled in the usual way, a liquid is obtained, which consists almost entirely of unaltered carbon disulphide ; but when this is allowed to evaporate spontaneously, a small quantity of a crystalline substance is left, which has the appearance and properties of carbon tetrabromide. The length of time required for this reaction, and the very small relative amount of substance obtained, would, however, render this Published on 01 January 1871. Downloaded by Brown University 25/10/2014 10:39:25. quite inapplicable as a process for the preparation of the tetra- bromide. Action of Bromine on Carbon Disdphide in, presence of Certain Bromides. -

1997-11-12 Carbon Disulfide As Federal Hazardous Air Pollutant
CARBON DISULFIDE Carbon disulfide is a federal hazardous air pollutant and was identified as a toxic air contaminant in April 1993 under AB 2728. CAS Registry Number: 75-15-0 CS2 Molecular Formula: CS2 Carbon disulfide is a highly refractive, mobile, and very flammable liquid. The purest distillates have a sweet odor. However, the usual commercial and reagent grades of carbon disulfide are foul smelling. It burns with a blue flame to form carbon dioxide and sulfur dioxide. Liquid carbon disulfide will attack some forms of plastics, rubber, and coatings but is non- corrosive to most commercial structural materials at ordinary temperatures. It is miscible in water, alcohol, oils, chloroform, and ether (Merck, 1989). Physical Properties of Carbon Disulfide Synonyms: carbon bisulfide; carbon disulphide; carbon sulfide; sulphocarbonic anhydride; sulphuret of carbon; dithiocarbonic anhydride Molecular Weight: 76.14 Boiling Point: 46.5 oC Melting Point: -111.5 oC Vapor Density: 2.67 (air =1) Flash Point: -30 oC (closed cup) Density/Specific Gravity: 1.2632 at 20/4 oC (water = 1) Critical Temperature: 280 oC Vapor Pressure: 297 mm Hg at 20 oC Log Octanol/Water Partition Coefficient: 1.70 - 4.16 Conversion Factor: 1 ppm = 3.11 mg/m3 (Howard, 1990; HSDB, 1991; Merck, 1989; U.S. EPA, 1994a) SOURCES AND EMISSIONS A. Sources Carbon disulfide is used in the preparation of rayon viscose fibers, and as a solvent for lipids, phosphorus, sulfur, selenium, bromine, iodine, rubber, resins, and waxes (Proctor et al, 1991). Carbon disulfide is also used in the manufacture of carbon tetrachloride, cellophane, flotation Toxic Air Contaminant Identification List Summaries - ARB/SSD/SES September 1997 193 Carbon Disulfide agents, xanthogenates, and numerous other chemicals (HSDB, 1991; Sax, 1987).