Epa Hazardous Waste Codes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methyl Ethyl Ketone (2-Butanone)
Methyl Ethyl Ketone (2-Butanone) 78-93-3 Hazard Summary Methyl ethyl ketone is used as a solvent. Acute (short-term) inhalation exposure to methyl ethyl ketone in humans results in irritation to the eyes, nose, and throat. Limited information is available on the chronic (long-term) effects of methyl ethyl ketone in humans. Chronic inhalation studies in animals have reported slight neurological, liver, kidney, and respiratory effects. No information is available on the developmental, reproductive, or carcinogenic effects of methyl ethyl ketone in humans. Developmental effects, including decreased fetal weight and fetal malformations, have been reported in mice and rats exposed to methyl ethyl ketone via inhalation and ingestion. EPA has classified methyl ethyl ketone as a Group D, not classifiable as to human carcinogenicity. Please Note: The main sources of information for this fact sheet are EPA's Health Effects Assessment for Methyl Ethyl Ketone (1) and EPA's Integrated Risk Information System (IRIS) (6), which contains information on inhalation chronic toxicity of methyl ethyl ketone and the RfC and oral chronic toxicity and the RfD. Uses The primary use of methyl ethyl ketone is as a solvent in processes involving gums, resins, cellulose acetate, and cellulose nitrate. (1) Methyl ethyl ketone is also used in the synthetic rubber industry, in the production of paraffin wax, and in household products such as lacquer and varnishes, paint remover, and glues. (1) Sources and Potential Exposure Methyl ethyl ketone has been detected in both indoor and outdoor air. Methyl ethyl ketone can be produced in outdoor air by the photooxidation of certain air pollutants, such as butane and other hydrocarbons. -

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Methyl Isopropyl Ketone Safety Data Sheet According to Federal Register / Vol
Methyl isopropyl ketone Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 05/25/2015 Version: 1.0 SECTION 1: Identification 1.1. Identification Product form : Substance Substance name : Methyl isopropyl ketone CAS-No. : 563-80-4 Product code : (US) W1814 Formula : C5H10O Synonyms : 3-Methylbutane-2-one / Isopropyl methyl ketone / Methyl-2-butanone, 3- / 2-Acetylpropane / 3- Methylbutanone-2 / 3-Methylbutanone / 3-Methylbutan-2-one / 3-Methyl-2-butanone / 2- Butanone, 3-methyl- / Butan-2-one, 3-methyl- 1.2. Recommended use and restrictions on use No additional information available 1.3. S upplie r Synerzine 5340 Hwy 42 S Ellenwood, Georgia 30294 - USA T 404-524-6744 - F 404-577-1651 [email protected] - www.synerzine.com 1.4. Emergency telephone number Emergency number : Infotrac 1-800-535-5053 (Contract# 102471) Dial +1-352-323-3500 when outside the US SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mi xt ure GHS -US classification Flammable liquids Category H225 Highly flammable liquid and vapour 2 Specific target organ toxicity H336 May cause drowsiness or dizziness (single exposure) Category 3 Hazardous to the aquatic H402 Harmful to aquatic life environment - Acute Hazard Category 3 Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS-US labeling Hazard pictograms (GHS-US) : Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H225 - Highly flammable liquid and vapour H336 - May cause drowsiness or dizziness H402 - Harmful to aquatic life Precautionary statements (GHS-US) : P210 - Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. -

Toxicological Review of Chloral Hydrate (CAS No. 302-17-0) (PDF)
EPA/635/R-00/006 TOXICOLOGICAL REVIEW OF CHLORAL HYDRATE (CAS No. 302-17-0) In Support of Summary Information on the Integrated Risk Information System (IRIS) August 2000 U.S. Environmental Protection Agency Washington, DC DISCLAIMER This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Note: This document may undergo revisions in the future. The most up-to-date version will be made available electronically via the IRIS Home Page at http://www.epa.gov/iris. ii CONTENTS—TOXICOLOGICAL REVIEW for CHLORAL HYDRATE (CAS No. 302-17-0) FOREWORD .................................................................v AUTHORS, CONTRIBUTORS, AND REVIEWERS ................................ vi 1. INTRODUCTION ..........................................................1 2. CHEMICAL AND PHYSICAL INFORMATION RELEVANT TO ASSESSMENTS ..... 2 3. TOXICOKINETICS RELEVANT TO ASSESSMENTS ............................3 4. HAZARD IDENTIFICATION ................................................6 4.1. STUDIES IN HUMANS - EPIDEMIOLOGY AND CASE REPORTS .................................................6 4.2. PRECHRONIC AND CHRONIC STUDIES AND CANCER BIOASSAYS IN ANIMALS ................................8 4.2.1. Oral ..........................................................8 4.2.2. Inhalation .....................................................12 4.3. REPRODUCTIVE/DEVELOPMENTAL STUDIES ..........................13 -

Chloral Hydrate and Paraldehyde As Drugs of Addiction
Sept., 1932J CHLORAL HYDRATE DRUG HABIT : CHOPRA & SINGH CHOPRA 481 certain parts of the Punjab. This is not the outcome of the use of the drug in the treatment Original Articles of insomnia, but is due to entirely different causes. Until a few years ago in that province potable country-made spirits were allowed to CHLORAL HYDRATE AND PARALDE' be sold to retail dealers in bulk and the vendors HYDE AS DRUGS OF ADDICTION bottled the liquor themselves. Some of these ingenious people conceived the idea of diluting R. N. m.d. By CHOPRA, m.a., (Cantab.) the spirit and adding small quantities of chloral I.M.S. LIEUTENANT-COLONEL, hydrate to make up for the loss in its potency and which would result from dilution. The know- GURBAKHSH SINGH CHOPRA, m.b., b.s. ledge that the drug had hypnotic and narcotic was obtained from the (Drug Addiction Inquiry, Indian Research Fund effects undoubtedly Association) medical profession and compounders work- in It was further learnt that Series No. 15 ing dispensaries. the effects chloral hydrate in many Chloral produced by hydrate and paraldehyde belong to resemble those by alcohol, the of ways produced group drugs known as soporifics or especially when the latter is taken in large The chief use of hypnotics. this class of drugs quantities. When the two articles are taken is in the treatment of one insomnia, of the together they act in a manner synergistic to worst evils of modern times from which man- each other and in this way the effect of either kind can suffer. -

Past Use of Chlordane, Dieldrin, And
The Hazard Evaluation and Emergency Response Office (HEER Office) is part of the Hawai‘i Department of Health (HDOH) Environmental Health Administration, whose mission is to protect human health and the environment. The HEER Office provides leadership, support, and partnership in preventing, planning for, responding to, and enforcing environmental laws relating to releases or threats of releases of hazardous substances. Past Use of Chlordane, Dieldrin, and other Organochlorine Pesticides for Termite Control in Hawai‘i: Safe Management Practices around Treated Foundations or during Building Demolition This fact sheet provides building owners, demolition and construction contractors, developers, realtors, and others with an overview of the potential environmental concerns associated with the past use of organochlorine termiticides (pesticides used to control termites) in Hawai‘i. In addition, this fact sheet discusses methods for reducing exposure to organochlorine termiticides during building demolition or around the foundations of treated buildings and identifies resources for further information. What are organochlorine termiticides? Organochlorine termiticides are a group of pesticides that were used for termite control in and around wooden buildings and homes from the mid-1940s to the late 1980s. These organochlorine pesticides included chlordane, aldrin, dieldrin, heptachlor, and dichlorodiphenyltrichloroethane (DDT). They were used primarily by pest control operators in Hawaii’s urban areas, but also by homeowners, the military, the state, and counties to protect buildings against termite damage. In the 1970s and 1980s, the U.S. Environmental Protection Agency (EPA) banned all uses of these organochlorine pesticides except for heptachlor, which can be used today only for control of fire ants in underground power transformers. -

' ' T UNITED' STATES PATENT 'V OFFICE
PatentedUNITED’ Apr.v22,_,>1947'-" STATES > PATENT' ‘ 'v TOFFICE ‘2,419,488 " PRODUCTION OF MoNocnLoRo DERIVA- ‘ ~ ' 'rrvns 0F UNSATUBATED NITRILES - ' Harris A. Dutcher, Borg'cr', ‘_Tex_., asslgnor 'to_ 4 Phillips Petroleum Company,.a corporation of ‘.Delaware> f . ' ' > v No Drawing. Application June 5,1944, I, > Serial No. 538,880 ‘ , ' 11 Claims. (01. zed-464$ , . 2 . The present invention relates to the production - Heretofore, a-chloroacrylonitrile has been pro of chlorine derivatives of unsaturated nitriles by ' duced as a by-product oi.‘ the direct substitutive the reaction of acetylene or acetylenic hydrocar- chlorination of acrylonitrile in the vapor phase bons with cyanogen chloride. More particularly over active carbon at a temperature between ap the invention relates to the production of 3- 5 proximately 200° and approximately 550° C. chloroacrylonitrile - ‘ . (Long, U. S. Patent No. 2,231,363). The process (Z-chloroviny] cyanide,‘ C1__CH___CH_CN) yields 2-chloroacrylonitrile as the principal prod uct. vNo other methods for the production of 3 rgldsl?ltlgfesmigoilxgr196ii?ggtggesagty‘gfgtg; chloroacrylonitrile or other monochloro deriva acetylenic hydrocarbons and cyanogen chloride 10 tives of unsaturatednitriles are known. ‘ ' ’ I have found that acetylene and acetylenic hy is 2281322232”; ‘13355;:ffgléetggesggtdilrgggi? . drocarbons may be reacted with cyanogen halides . I . v to produce.monohalogen-substituted unsaturated monochloro derivatives of unsaturated mtnles, . 1 such as 3-chloroacrylonitrile by there'action of- 1 mtr?es' The reactlon 1.5 facihtated ‘by certain ’ - » >15 catalysts which are referred to more speci?cally acetylene and cyanogen chloride. , hereinafter _ provideAnother a categlyst ob'ect (1201‘f thpfoggtiiirgtthevggg?lo?rg- e ‘n ' ' ‘ o Cyanogen' ‘ihlmd‘?. 157a readny.condens1ble. -

Argonne Report.Pdf
CONTENTS NOTATION ........................................................................................................................... xi ABSTRACT ........................................................................................................................... 1 1 INTRODUCTION ........................................................................................................... 5 1.1 Overview of the Emergency Response Guidebook ................................................ 5 1.2 Organization of this Report ..................................................................................... 7 2 GENERAL METHODOLOGY ....................................................................................... 9 2.1 TIH List ................................................................................................................... 10 2.1.1 Background ................................................................................................. 10 2.1.2 Changes in the TIH List for the ERG2012 ................................................. 11 2.2 Shipment and Release Scenarios ............................................................................ 11 2.2.1 Shipment Profiles ........................................................................................ 12 2.2.2 Treatment of Chemical Agents ................................................................... 14 2.3 Generics, Mixtures, and Solutions .......................................................................... 17 2.4 Analysis of Water-Reactive -
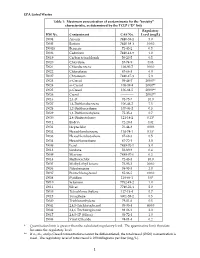
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Measurement Technique for the Determination of Photolyzable
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 102, NO. D13, PAGES 15,999-16,004,JULY 20, 1997 Measurement techniquefor the determination of photolyzable chlorine and bromine in the atmosphere G. A. Impey,P. B. Shepson,• D. R. Hastie,L. A. Bartie• Departmentof Chemistryand Centre for AtmosphericChemistry, York University,Toronto, Ontario, Canada Abstract. A techniquehas been developed to enablemeasurement of photolyzablechlorine and bromineat tracelevels in the troposphere.In thismethod, ambient air is drawnt•ough a cylindricalflow cell, whichis irradiatedwith a Xe arc lamp. In the reactionvessel of the photoactivehalogen detector (PHD), photolyrically active molecules Clp (including C12, HOC1, C1NO,C1NO2, and C1ONO2) and Brp (including Br2, HOBr, BrNO, BrNO2, and BrONO2) are photolyzed,and the halogenatoms produced react with properieto form stablehalogenated products.These products are thensampled and subsequently separated and detected by gas chromatography.The systemis calibratedusing low concentrationmixtures of C12and Br2 in air from commerciallyavailable permeation sources. We obtaineddetection limits of 4 pptv and 9 pptv as Br2 andC12, respectively, for 36 L samples. 1. Introduction (or C12)in the Arctic, largely as a result of the lack of suitable analyticalmethodologies. This paperreports the developmentof The episodicdestruction of groundlevel ozonein the Arctic at a measurementtechnique for the determinationof rapidly sunriseis a phenomenonthat hasbeen observed for many years. photolyzingchlorine (referred to hereas Clp) and bromine (Brp) With the onsetof polar sunrise,ozone levels are often observed speciesat pansper trillion by volume(pptv) mixingratios in the to drop from a backgroundconcentration of •40 ppbv to almost atmosphere.Impey et al. [this issue]discuss the resultsobserved zero on a timescaleof a day or less [Barrie et al., 1988] for from a field studyconducted in the Canadianhigh Arctic at Alert, periodsof 1-10 days.