SROC Annex V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

CAS REGISTRY: Finding CAS Registry Numbers
STN® Quick Reference Card CAS REGISTRYSM: Finding CAS Registry Numbers® When you know the Substance Name Field Use Example /CN Use when you know the complete => E BENZOIC ACID/CN Chemical substance name. EXPAND first to => E “BICYCLO(2.2.1)HEPTANE”/CN Name determine if the name is in the database. Basic Index Use when you know segments of the => S FLUOROMETHYL name or don’t know how the name => S “2,2’” (W) BIPYRID? segments go together. => S PYRIDINE (XW) DICARBOXY? /CNS Use when you want to search for a => S ?MYCIN?/CNS Chemical Name character string embedded in a trade => S ?QUAT/CNS Segments name. Use left and right truncation to search for an embedded character string. Use when you want to require that a => S CHLOROPHENYL/CNS name segment is not part of a larger => S HEXANEDIOIC (XW) segment. DIMETHYL/CNS /HP Use when you want to restrict the search => S 1-PROPANOL/HP Heading to a Heading Parent from a CA index => S 2-PYRIDINECARBO?/HP Parent name. /INS.HP Use when you want to restrict the search => S (PYRIDINE (XW) Index Name to name segments from the Heading CARBONITRIL?)/INS.HP part of a CA index name. Segments - Parent => S FLUOROMETHYL/INS.HP Heading Parent /INS.NHP Use when you want to restrict the search => S MORPHOL?/INS.NHP Index Name to name segments from the non- => S FLUOROMETHYL/INS.NHP Segments - part of a CA index Heading-Parent Non-Heading name. Parent /ONS Use when you want to restrict the search => S VINCAM?/ONS Other Name to name segments from names other => S (INDOLE(XW)AMIN?)/ONS Segments than CA index names such as semi- systematic names, trade names, common names, etc. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -
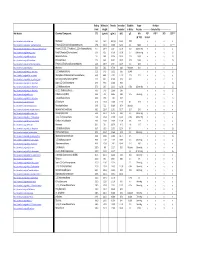
Detectable Compounds by MIP.Pdf
Boiling Molecular Density Ionization Solubility Vapor Analytes Point Weight Potential in Water Pressure ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐Detected By‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Wiki Website Chemical Compound: (oC) (g/mol) (g/mL) (eV) g/L kPa PID* FID** XSD ECD*** @ ~25C 10.6 eV https://en.wikipedia.org/wiki/Methane . Methane ‐161 16.0 .657 g/L 12.61 0.02 n y n n https://en.wikipedia.org/wiki/Dichlorodifluoromethane . Freon 12 (Dichlorodifluoromethane) ‐29.8 120.9 1.486 12.31 0.3 568.0 n y y LS**** https://en.wikipedia.org/wiki/1,1,2‐Trichloro‐1,2,2‐trifluoroethane . Freon 113 (1,1,2‐Trichloro‐1,2,2‐trifluoroethane) 47.7 187.4 1.564 11.78 0.17 285mm Hg n y y y https://en.wikipedia.org/wiki/Vinyl_chloride . Vinyl Chloride (Chloroethene) ‐13.0 62.5 0.910 10.00 2.7 2580mm Hg y y y LS https://en.wikipedia.org/wiki/Bromomethane . Bromomethane 3.6 94.9 3.974 10.53 17.5 190.0 n y y LS https://en.wikipedia.org/wiki/Chloroethane .. Chloroethane 12.3 64.5 0.901 10.97 5.74 134.6 n y y LS https://en.wikipedia.org/wiki/Trichlorofluoromethane . Freon 11 (Trichlorofluoromethane) 23.8 137.4 1.494 11.77 1.1 89.0 n y y y https://en.wikipedia.org/wiki/Acetone . Acetone 56.5 58.1 0.790 9.69 Miscible 30.6 y y n n https://en.wikipedia.org/wiki/1,1‐Dichloroethene . 1,1‐Dichloroethene 32.0 97.0 1.213 9.65 0.04% y y y LS https://en.wikipedia.org/wiki/Dichloromethane . -

REGISTRY Database Summary Sheet (DBSS)
SM REGISTRY/ZREGISTRY (CAS REGISTRY ) Subject • All types of inorganic and organic substances, including alloys, coordination Coverage compounds, minerals, mixtures, polymers, salts, high throughput screening (HTS) compounds as well as nucleic acid and protein sequences • Substances included in REGISTRY meet the following criteria: - Identified by CAS as coming from a reputable source, including but not limited to patents, journals, chemical catalogs, and selected substance collections on the web - Described in largely unambiguous terms - Characterized by physical methods or described in a patent document example or claim - Consistent with the laws of atomic covalent organization • Experimental and predicted property data and tags and spectra data File Type Numeric, Structure Features Alerts (SDIs) Biweekly In addition, SMARTracker, an automatic crossfile current-awareness search, (SDI XFILE) using a REGISTRY search profile in CA, HCA, ZCA, CAplus, HCAplus or ZCAplus may be run weekly or biweekly (weekly is the default). CAS Registry Keep & Share SLART Number® Identifiers ® Learning Database STN Easy Structures Record • CAS Registry Numbers • Experimental and predicted property Content • CA index names and commonly used data and tags chemical names and trade names • Spectra • Molecular formulas • List of databases and regulatory listings • Structure diagrams containing information on the substances • Sequence data • Super roles and document type SM • Alloy composition tables information from CAplus • Classes for polymers • Displayable information for 10 most recent references for substances • Ring analysis data File Size More than 140 million organic and inorganic substances; more than 71.1 million sequences (4/18) Coverage Early 1800s to the present Updates Daily Language English Database Chemical Abstracts Service Producer 2540 Olentangy River Road P.O. -

Exposure Investigation Protocol: the Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH
Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan Exposure Investigation Protocol: The Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH MDCH/ATSDR - 2004 Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan TABLE OF CONTENTS OBJECTIVE/PURPOSE..................................................................................................... 3 RATIONALE...................................................................................................................... 4 BACKGROUND ................................................................................................................ 5 AGENCY ROLES .............................................................................................................. 6 ESTABLISHING CRITERIA ............................................................................................ 7 “Odor Events”................................................................................................................. 7 Comparison Values......................................................................................................... 7 METHODS ....................................................................................................................... 12 Instantaneous (“Grab”) Air Sampling........................................................................... 12 Continuous Air Monitoring.......................................................................................... -

Antimony Pentachloride Apc
ANTIMONY PENTACHLORIDE APC CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: 99+% Common Synonyms Liquid Colorless to brown Unpleasant odor Not flammable 7.2 Storage Temperature: Ambient Antimony (V) chloride 4.2 Flammable Limits in Air: Not flammable Antimony perchloride 7.3 Inert Atmosphere: No requirement 4.3 Fire Extinguishing Agents: Not pertinent Sinks in water. Irritating vapor is produced. Freezing point is 37°F. 7.4 Venting: Pressure-vacuum 4.4 Fire Extinguishing Agents Not to Be 7.5 IMO Pollution Category: Currently not available Avoid contact with liquid. Keep people away. Used: Do not use water or foam on Wear rubber overclothing (including gloves). adjacent fires. 7.6 Ship Type: Currently not available Stop discharge if possible. 4.5 Special Hazards of Combustion 7.7 Barge Hull Type: Currently not available Evacuate. Products: Not pertinent Isolate and remove discharged material. 4.6 Behavior in Fire: Irritating fumes of Notify local health and pollution control agencies. 8. HAZARD CLASSIFICATIONS hydrogen chloride given off when water Protect water intakes. or foam is used to extinguish adjacent 8.1 49 CFR Category: Corrosive material fire. 8.2 49 CFR Class: 8 Not flammable. 4.7 Auto Ignition Temperature: Not pertinent Fire POISONOUS GASES ARE PRODUCED WHEN HEATED. 8.3 49 CFR Package Group: II DO NOT USE WATER ON ADJACENT FIRES. 4.8 Electrical Hazards: Not pertinent 8.4 Marine Pollutant: No 4.9 Burning Rate: Not pertinent 8.5 NFPA Hazard Classification: Exposure Call for medical aid. 4.10 Adiabatic Flame Temperature: Not pertinent Category Classification VAPOR Health Hazard (Blue)......... -

Multivalent Metals and Polyatomic Ions 1
Name Date Comprehension Section 4.2 Use with textbook pages 189–193. Multivalent metals and polyatomic ions 1. Define the following terms: (a) ionic compound (b) multivalent metal (c) polyatomic ion 2. Write the formulae and names of the compounds with the following combination of ions. The first row is completed to help guide you. Positive ion Negative ion Formula Compound name (a) Pb2+ O2– PbO lead(II) oxide (b) Sb4+ S2– (c) TlCl (d) tin(II) fluoride (e) Mo2S3 (f) Rh4+ Br– (g) copper(I) telluride (h) NbI5 (i) Pd2+ Cl– 3. Write the chemical formula for each of the following compounds. (a) manganese(II) chloride (f) vanadium(V) oxide (b) chromium(III) sulphide (g) rhenium(VII) arsenide (c) titanium(IV) oxide (h) platinum(IV) nitride (d) uranium(VI) fluoride (i) nickel(II) cyanide (e) nickel(II) sulphide (j) bismuth(V) phosphide 68 MHR • Section 4.2 Names and Formulas of Compounds © 2008 McGraw-Hill Ryerson Limited 0056_080_BCSci10_U2CH04_098461.in6856_080_BCSci10_U2CH04_098461.in68 6688 PDF Pass 77/11/08/11/08 55:25:38:25:38 PPMM Name Date Comprehension Section 4.2 4. Write the formulae for the compounds formed from the following ions. Then name the compounds. Ions Formula Compound name + – (a) K NO3 KNO3 potassium nitrate 2+ 2– (b) Ca CO3 + – (c) Li HSO4 2+ 2– (d) Mg SO3 2+ – (e) Sr CH3COO + 2– (f) NH4 Cr2O7 + – (g) Na MnO4 + – (h) Ag ClO3 (i) Cs+ OH– 2+ 2– (j) Ba CrO4 5. Write the chemical formula for each of the following compounds. (a) barium bisulphate (f) calcium phosphate (b) sodium chlorate (g) aluminum sulphate (c) potassium chromate (h) cadmium carbonate (d) calcium cyanide (i) silver nitrite (e) potassium hydroxide (j) ammonium hydrogen carbonate © 2008 McGraw-Hill Ryerson Limited Section 4.2 Names and Formulas of Compounds • MHR 69 0056_080_BCSci10_U2CH04_098461.in6956_080_BCSci10_U2CH04_098461.in69 6699 PDF Pass77/11/08/11/08 55:25:39:25:39 PPMM Name Date Comprehension Section 4.2 Use with textbook pages 186–196. -

Synthesis of Bromochloromethane Using Phase Transfer Catalysis
1 SYNTHESIS OF BROMOCHLOROMETHANE USING PHASE TRANSFER CATALYSIS By LANCELOT LUCRETIUS BROOKS Baccalaureus Scientiae Honores-Chemistry, Nelson Mandela Metropolitan University A dissertation submitted in fulfillment of the requirements for the Masters Degree in Chemistry In the Faculty of Science at the NELSON MANDELA METROPOLITAN UNIVERSITY Nov. 2011 Promoter : Dr G. Dugmore Co-Promoter : Prof B. Zeelie 2 DECLARATION I, Lancelot Brooks, hereby declare that the above-mentioned treatise is my own work and that it has not previously been submitted for assessment to another University, or for another qualification. ……………………………….. ……………………….. Mr. L.L. Brooks Date 3 ACKNOWLEDGEMENTS To my promoters Dr. Gary Dugmore, and Prof. Ben Zeelie for their invaluable input, help and guidance. To NRF and NMMU for financial assistance To my parents and brothers for their love and support To Peter, Batsho, Unati, and friends in NMMU chemistry research laboratory, thank you guys. To my dearest fiancée, Natasha, a very special thank you for always being there and supporting me. Love you angel. “And we know that all things work together for good to those who love God, to those who are called according to His purpose” -Romans 8:28. 4 TABLE OF CONTENTS DECLARATION……………………………………………………………………. 2 ACKNOWLEDGEMENTS……………………………………………………………. 3 TABLE OF CONTENTS………………………………………………………………. 4 LIST OF FIGURES…………………………………………………………………….. 8 LIST OF TABLES……………………………………………………………………… 9 LIST OF EQUATIONS………………………………………………………………… 11 SUMMARY……………………………………………………………………………… 12 CHAPTER 1…………………………………………………………………………….. 14 INTRODUCTION………………………………………………………………………. 14 1.1. Technology of leather production……………………………………………….. 14 1.2. Synthesis of TCMTB……………………………………………………………… 17 1.3. Bromine……………………………………………………………………………. 20 1.3.1. Overview……………………………………………………………. 20 1.3.2. Applications of bromine compounds…………..…………………. 22 1.3.2.1. Photography……………………………………………… 22 1.3.2.2. -

Supplement to the Role of Internal Standards and Their Interaction With
Supplement to: Hiatt, M., “The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations,” Int. J. of Environ. Anal. Chem., 2010 90:8 591-604. Please note that this is a non-copyrighted web version of supplemental information belonging to the above-cited journal article. There are formatting and page-numbering differences between this web version and the actual published version. There are also minor content differences. This version can be accessed at: http://www.epa.gov/nerlesd1/chemistry/vacuum/reference/pubs.htm This publication is also available from Informaworld: http://www.informaworld.com/smpp/content~db=all~content=a921288588 Web version, 27 April 2010 1 Supplement to: The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations Michael H. Hiatt U.S. Environmental Protection Agency, National Exposure Research Laboratory Environmental Sciences Division. P.O. Box 93478, Las Vegas, Nevada 89193-3478 Phone: 702 798 2381. Fax: 702 798 2142. E-mail: [email protected]. Supplementary Information Data that was too detailed for “The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations” is presented as supplemental information. This information includes each analyte by matrix and includes the accuracy evaluation, analyte results impacted by criteria, and results from varying spike equilibration times. The list of internal standards and their chemical properties are presented in Table S1 while the surrogates are listed in Table S2. Tables of results by analyte are included as Tables S3-8. These tables include the data by matrix and combined. -

Finding Refrigerant Leaks with the Chempro100i
Application Note: 108 Finding Refrigerant Leaks with the ChemPro100i The ChemPro100i is a great sniffer for halocarbon refrigerants, often generically referred to as “Freon” because of its multiple sensors and broad sniffing capability. As refrigerant sniffers are only carried by a small subset of first responders, the ChemPro100i can fill the role for those that don’t have a refrigerant sniffer. As a bonus, if someone suspects that it may be a refrigerant leak, and it turns out to be something else, the wide range of detectable gases and vapors seen by the ChemPro100i means that it will most likely find the unexpected gas/vapor too. The ChemPro100i’s Sensors The ChemPro100i uses a suite of seven sensors including an aspirated Ion Mobility Spectroscopy (IMS), five metal oxide sensors and a field effect sensor to detect, characterize, and even identify, some gases and vapors. Using this suite of sensors, the ChemPro100i can find halocarbon refrigerant leaks using its “Trend” or “sniffer” screen. As one gets closer to the refrigerant leak, the trend line will Using other Sniffers for increase. This is a non-quantifiable reading Refrigerants that does not directly correlate with parts per Most dedicated refrigerant detectors use a million (ppm), but the fast response of the Metal Oxide Sensor (MOS) that is doped to ChemPro100i to refrigerants provides one with be relatively specific to the halogenated the means of quickly finding a refrigerant leak. hydrocarbons (halogens) that are the hallmark of most refrigerants. MOS sensors Increase in the Trend line leads you to the leak are non-linear and not suitable for quantification of refrigerants, but they provide sensitive and fast response to halocarbon refrigerants. -

UV Spectroscopic Determination of the Chlorine Monoxide (Clo)/Chlorine Peroxide (Cloocl) Thermal Equilibrium Constant
Atmos. Chem. Phys., 19, 6205–6215, 2019 https://doi.org/10.5194/acp-19-6205-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. UV spectroscopic determination of the chlorine monoxide (ClO) = chlorine peroxide (ClOOCl) thermal equilibrium constant J. Eric Klobas1,2 and David M. Wilmouth1,2 1Harvard John A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA 2Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA Correspondence: J. Eric Klobas ([email protected]) Received: 22 October 2018 – Discussion started: 29 October 2018 Revised: 19 April 2019 – Accepted: 24 April 2019 – Published: 10 May 2019 Abstract. The thermal equilibrium constant between the net V 2O3 ! 3O2 (R5) chlorine monoxide radical (ClO) and its dimer, chlorine peroxide (ClOOCl), was determined as a function of tem- Within this cycle, the equilibrium governing the partitioning perature between 228 and 301 K in a discharge flow ap- of ClO and ClOOCl in Reaction (R1) is defined as follows. paratus using broadband UV absorption spectroscopy. A [ClOOCl] third-law fit of the equilibrium values determined from Keq D (1) [ClO]2 the experimental data provides the expression Keq D 2:16 × 10−27e.8527±35 K=T / cm3 molecule−1 (1σ uncertainty). A This thermal equilibrium is a key parameter that deter- second-law analysis of the data is in good agreement. From mines the nighttime partitioning of active chlorine in the the slope of the van’t Hoff plot in the third-law analy- winter–spring polar vortex.