A Synthetic Lethal Screen Identifies ATR-Inhibition As a Novel Therapeutic Approach for POLD1-Deficient Cancers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Supplementary Text and Figures.Pdf
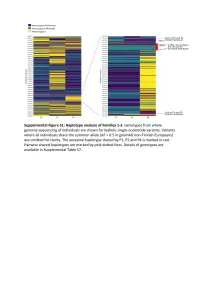
Supplemental Figure S2. PRIM1 immunoblots (20-75 kDa). (A) Corresponds to Figure 2D, with additional bands seen in P2 at 75 kDa and 25 kDa. (B) Independent experiment with cell lysates from C and P2 in which additional bands at 25 kDa not evident. (C) Corresponds to Figure 2D. No additional band visible at 25 kDa. (D) Validation of monoclonal antibody (8G10) with siRNA to PRIM1 in HeLa cells. siLUC, luciferase negative control. siPRIM1, siRNA targeting PRIM1 transcript. Vinculin used as a loading control. Supplemental Figure S3: Predicted destabilisation of PRIM1 by the C301R substitution. (A) Cysteine 301 lies in a buried hydrophobic region in PRIM1. DNA primase dimer crystal structure (PDB: 4BPU). PRIM1 residues shaded according to solvent accessibility. Cysteine 301, substituted to arginine in P5, lies in a buried hydrophobic region. (B) Cys301 is evolutionarily conserved in vertebrates. Neither arginine or other large or charged amino acids are observed at this position in other species. (C) The C301R substitution observed in P5 is predicted to lead to destabilisation of all available PRIM1 crystal structures. In contrast, substitution with leucine or threonine, as observed in some orthologous proteins, is not predicted to lead to destabilisation. Data points, predicted changes in free-energy (ΔΔG) from FoldX plotted for each of 14 available crystal structures. (D) Immunoblotting of RPE1 cells transfected with dual reporter constructs (as described in Figure 3B and Materials and Methods) confirms production of PRIM1-GFP and FLAG-SR at expected molecular weights and shows reduced protein levels for the C301R variant. n=2 experiments shown. C301R protein levels relative to WT after normalization to actin loading control indicated underneath the blot. -

Gene Expression Profiling Analysis Contributes to Understanding the Association Between Non-Syndromic Cleft Lip and Palate, and Cancer
2110 MOLECULAR MEDICINE REPORTS 13: 2110-2116, 2016 Gene expression profiling analysis contributes to understanding the association between non-syndromic cleft lip and palate, and cancer HONGYI WANG, TAO QIU, JIE SHI, JIULONG LIANG, YANG WANG, LIANGLIANG QUAN, YU ZHANG, QIAN ZHANG and KAI TAO Department of Plastic Surgery, General Hospital of Shenyang Military Area Command, PLA, Shenyang, Liaoning 110016, P.R. China Received March 10, 2015; Accepted December 18, 2015 DOI: 10.3892/mmr.2016.4802 Abstract. The present study aimed to investigate the for NSCL/P were implicated predominantly in the TGF-β molecular mechanisms underlying non-syndromic cleft lip, signaling pathway, the cell cycle and in viral carcinogenesis. with or without cleft palate (NSCL/P), and the association The TP53, CDK1, SMAD3, PIK3R1 and CASP3 genes were between this disease and cancer. The GSE42589 data set found to be associated, not only with NSCL/P, but also with was downloaded from the Gene Expression Omnibus data- cancer. These results may contribute to a better understanding base, and contained seven dental pulp stem cell samples of the molecular mechanisms of NSCL/P. from children with NSCL/P in the exfoliation period, and six controls. Differentially expressed genes (DEGs) were Introduction screened using the RankProd method, and their potential functions were revealed by pathway enrichment analysis and Non-syndromic cleft lip, with or without cleft palate (NSCL/P) construction of a pathway interaction network. Subsequently, is one of the most common types of congenital defect and cancer genes were obtained from six cancer databases, and affects 3.4-22.9/10,000 individuals worldwide (1). -

PRIM1 Polyclonal ANTIBODY Catalog Number:10773-1-AP 2 Publications
For Research Use Only PRIM1 Polyclonal ANTIBODY www.ptglab.com Catalog Number:10773-1-AP 2 Publications Catalog Number: GenBank Accession Number: Purification Method: Basic Information 10773-1-AP BC005266 Antigen affinity purification Size: GeneID (NCBI): Recommended Dilutions: 150UL , Concentration: 133 μg/ml by 5557 WB 1:500-1:1000 Bradford method using BSA as the Full Name: IP 0.5-4.0 ug for IP and 1:200-1:1000 standard; primase, DNA, polypeptide 1 (49kDa) for WB Source: IHC 1:20-1:200 Calculated MW: IF 1:50-1:500 Rabbit 50 kDa Isotype: Observed MW: IgG 50 kDa Immunogen Catalog Number: AG1124 Applications Tested Applications: Positive Controls: IF, IHC, IP, WB, ELISA WB : K-562 cells, HeLa cells, Sp2/0 cells Cited Applications: IP : HeLa cells, IHC IHC : human lymphoma tissue, Species Specificity: human, mouse, rat IF : HeLa cells, Cited Species: mouse Note-IHC: suggested antigen retrieval with TE buffer pH 9.0; (*) Alternatively, antigen retrieval may be performed with citrate buffer pH 6.0 DNA replication in human cells is initiated by a complex apparatus containing a DNA polymerase-alpha/primase Background Information complex. Primase synthesizes oligoribonucleotides that serve as primers for the initiation of DNA synthesis. It plays a role in both the initiation of DNA replication and the synthesis of Okazaki fragments for lagging strand synthesis. PRIM1 is a subnuit of this complex. Notable Publications Author Pubmed ID Journal Application Pierre Kunz 30986223 PLoS One IHC Liam C Hunt 31365869 Cell Rep Storage: Storage Store at -20°C. Stable for one year after shipment. -

Consequences and Resolution of Transcription–Replication Conflicts
life Review Consequences and Resolution of Transcription–Replication Conflicts Maxime Lalonde †, Manuel Trauner †, Marcel Werner † and Stephan Hamperl * Institute of Epigenetics and Stem Cells (IES), Helmholtz Zentrum München, 81377 Munich, Germany; [email protected] (M.L.); [email protected] (M.T.); [email protected] (M.W.) * Correspondence: [email protected] † These authors contributed equally. Abstract: Transcription–replication conflicts occur when the two critical cellular machineries respon- sible for gene expression and genome duplication collide with each other on the same genomic location. Although both prokaryotic and eukaryotic cells have evolved multiple mechanisms to coordinate these processes on individual chromosomes, it is now clear that conflicts can arise due to aberrant transcription regulation and premature proliferation, leading to DNA replication stress and genomic instability. As both are considered hallmarks of aging and human diseases such as cancer, understanding the cellular consequences of conflicts is of paramount importance. In this article, we summarize our current knowledge on where and when collisions occur and how these en- counters affect the genome and chromatin landscape of cells. Finally, we conclude with the different cellular pathways and multiple mechanisms that cells have put in place at conflict sites to ensure the resolution of conflicts and accurate genome duplication. Citation: Lalonde, M.; Trauner, M.; Keywords: transcription–replication conflicts; genomic instability; R-loops; torsional stress; common Werner, M.; Hamperl, S. fragile sites; early replicating fragile sites; replication stress; chromatin; fork reversal; MIDAS; G-MiDS Consequences and Resolution of Transcription–Replication Conflicts. Life 2021, 11, 637. https://doi.org/ 10.3390/life11070637 1. -

R‐Loop Formation During S Phase Is Restricted by Primpol‐Mediated
Published online: November 26, 2018 Article R-loop formation during S phase is restricted by PrimPol-mediated repriming Sasa Svikovic1, Alastair Crisp1, Sue Mei Tan-Wong2, Thomas A Guilliam3, Aidan J Doherty3, Nicholas J Proudfoot2, Guillaume Guilbaud1 & Julian E Sale1,* Abstract sequences pose a barrier to DNA synthesis in vivo and the conse- quences of their doing so are not well understood. During DNA replication, conflicts with ongoing transcription are It is well established that long repetitive tracts lead to problems frequent and require careful management to avoid genetic insta- with both replication and transcription. For example, a long tract of bility. R-loops, three-stranded nucleic acid structures comprising a polypurine–polypyrimidine (GAA)n repeats (in which n can exceed DNA:RNA hybrid and displaced single-stranded DNA, are important 1,500) is linked to the inherited neurodegenerative disorder Friedre- drivers of damage arising from such conflicts. How R-loops stall ich’s ataxia (Campuzano et al, 1996). These repeats can form H- replication and the mechanisms that restrain their formation DNA (Frank-Kamenetskii & Mirkin, 1995), a triplex DNA structure during S phase are incompletely understood. Here, we show in vivo able to block replication both in bacterial, yeast and human cells how R-loop formation drives a short purine-rich repeat, (GAA)10,to (Ohshima et al, 1998; Krasilnikova & Mirkin, 2004; Chandok et al, become a replication impediment that engages the repriming 2012), which can promote genetic instability of the repeat (Gerhardt activity of the primase-polymerase PrimPol. Further, the absence et al, 2016). Furthermore, these repetitive tracts are prone to form of PrimPol leads to significantly increased R-loop formation around R-loops (Groh et al, 2014), three-stranded nucleic acid structures in this repeat during S phase. -

In Utero Exposure to Chlordecone Affects Histone Modifications And
In utero exposure to chlordecone affects histone modifications and activates LINE-1 in cord blood Louis Legoff, Shereen Cynthia D’cruz, Katia Bouchekhchoukha, Christine Monfort, Christian Jaulin, Luc Multigner, Fatima Smagulova To cite this version: Louis Legoff, Shereen Cynthia D’cruz, Katia Bouchekhchoukha, Christine Monfort, Christian Jaulin, et al.. In utero exposure to chlordecone affects histone modifications and activates LINE- 1 in cord blood. Life Science Alliance, Life Science Alliance LLC, 2021, 4 (6), pp.e202000944. 10.26508/lsa.202000944. hal-03217292 HAL Id: hal-03217292 https://hal.archives-ouvertes.fr/hal-03217292 Submitted on 4 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Published Online: 9 April, 2021 | Supp Info: http://doi.org/10.26508/lsa.202000944 Downloaded from life-science-alliance.org on 4 May, 2021 Research Article In utero exposure to chlordecone affects histone modifications and activates LINE-1 in cord blood Louis Legoff1, Shereen Cynthia D’Cruz1, Katia Bouchekhchoukha1, Christine Monfort1, Christian Jaulin2, Luc Multigner1 , Fatima Smagulova1 Environmental factors can induce detrimental consequences into Epigenetic reprogramming during development is associated with a adulthood life. -

DNA Polymerases at the Eukaryotic Replication Fork Thirty Years After: Connection to Cancer
cancers Review DNA Polymerases at the Eukaryotic Replication Fork Thirty Years after: Connection to Cancer Youri I. Pavlov 1,2,* , Anna S. Zhuk 3 and Elena I. Stepchenkova 2,4 1 Eppley Institute for Research in Cancer and Allied Diseases and Buffett Cancer Center, University of Nebraska Medical Center, Omaha, NE 68198, USA 2 Department of Genetics and Biotechnology, Saint-Petersburg State University, 199034 Saint Petersburg, Russia; [email protected] 3 International Laboratory of Computer Technologies, ITMO University, 197101 Saint Petersburg, Russia; [email protected] 4 Laboratory of Mutagenesis and Genetic Toxicology, Vavilov Institute of General Genetics, Saint-Petersburg Branch, Russian Academy of Sciences, 199034 Saint Petersburg, Russia * Correspondence: [email protected] Received: 30 September 2020; Accepted: 13 November 2020; Published: 24 November 2020 Simple Summary: The etiology of cancer is linked to the occurrence of mutations during the reduplication of genetic material. Mutations leading to low replication fidelity are the culprits of many hereditary and sporadic cancers. The archetype of the current model of replication fork was proposed 30 years ago. In the sequel to our 2010 review with the words “years after” in the title inspired by A. Dumas’s novels, we go over new developments in the DNA replication field and analyze how they help elucidate the effects of the genetic variants of DNA polymerases on cancer. Abstract: Recent studies on tumor genomes revealed that mutations in genes of replicative DNA polymerases cause a predisposition for cancer by increasing genome instability. The past 10 years have uncovered exciting details about the structure and function of replicative DNA polymerases and the replication fork organization. -

Identification of Proteins Involved in the Maintenance of Genome Stability
Identification of Proteins Involved in the Maintenance of Genome Stability by Edith Hang Yu Cheng A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Department of Biochemistry University of Toronto ©Copyright by Edith Cheng2015 Identification of Proteins Involved in the Maintenance of Genome Stability Edith Cheng Doctor of Philosophy Department of Biochemistry University of Toronto 2015 Abstract Aberrant changes to the genome structure underlie numerous human diseases such as cancers. The functional characterization ofgenesand proteins that maintain chromosome stability will be important in understanding disease etiology and developing therapeutics. I took a multi-faceted approach to identify and characterize genes involved in the maintenance of genome stability. As biological pathways involved in genome maintenance are highly conserved in evolution, results from model organisms can greatly facilitate functional discovery in humans. In S. cerevisiae, I identified 47 essential gene depletions with elevated levels of spontaneous DNA damage foci and 92 depletions that caused elevated levels of chromosome rearrangements. Of these, a core subset of 15 DNA replication genes demonstrated both phenotypes when depleted. Analysis of rearrangement breakpoints revealed enrichment at yeast fragile sites, Ty retrotransposons, early origins of replication and replication termination sites. Together, thishighlighted the integral role of DNA replicationin genome maintenance. In light of my findings in S. cerevisiae, I identified a list of 153 human proteins that interact with the nascentDNA at replication forks, using a DNA pull down strategy (iPOND) in human cell lines. As a complementary approach for identifying human proteins involved in genome ii maintenance, I usedthe BioID techniqueto discernin vivo proteins proximal to the human BLM- TOP3A-RMI1-RMI2 genome stability complex, which has an emerging role in DNA replication progression. -

The DNA Polymerases of Drosophila Melanogaster
Fly ISSN: 1933-6934 (Print) 1933-6942 (Online) Journal homepage: https://www.tandfonline.com/loi/kfly20 The DNA polymerases of Drosophila melanogaster Steven J. Marygold, Helen Attrill, Elena Speretta, Kate Warner, Michele Magrane, Maria Berloco, Sue Cotterill, Mitch McVey, Yikang Rong & Masamitsu Yamaguchi To cite this article: Steven J. Marygold, Helen Attrill, Elena Speretta, Kate Warner, Michele Magrane, Maria Berloco, Sue Cotterill, Mitch McVey, Yikang Rong & Masamitsu Yamaguchi (2020): The DNA polymerases of Drosophilamelanogaster, Fly, DOI: 10.1080/19336934.2019.1710076 To link to this article: https://doi.org/10.1080/19336934.2019.1710076 © 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group. View supplementary material Published online: 14 Jan 2020. Submit your article to this journal Article views: 342 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=kfly20 FLY https://doi.org/10.1080/19336934.2019.1710076 REVIEW The DNA polymerases of Drosophila melanogaster Steven J. Marygold a, Helen Attrill a, Elena Speretta b, Kate Warner b, Michele Magrane b, Maria Berloco c, Sue Cotterill d, Mitch McVey e, Yikang Rong f, and Masamitsu Yamaguchig aFlyBase, Department of Physiology, Development and Neuroscience, University of Cambridge, Cambridge, UK; bUniProt, European Molecular Biology Laboratory, European Bioinformatics Institute (EMBL-EBI), Cambridgeshire, UK; cDipartimento di Biologia, -

DNA Primase Subunit 1 Deteriorated Progression of Hepatocellular
Zhu et al. Cell Biosci (2021) 11:42 https://doi.org/10.1186/s13578-021-00555-y Cell & Bioscience RESEARCH Open Access DNA primase subunit 1 deteriorated progression of hepatocellular carcinoma by activating AKT/mTOR signaling and UBE2C-mediated P53 ubiquitination Mengqi Zhu1,2,3†, Mengna Wu1†, Saiyan Bian1,2†, Qianqian Song4, Mingbing Xiao1, Hua Huang1, Li You1, Jianping Zhang1, Jie Zhang3, Chun Cheng2, Wenkai Ni1* and Wenjie Zheng1,3* Abstract Background: DNA primase subunit 1 (PRIM1) has been reported as a novel oncogene in several cancer types. How- ever, its roles in hepatocellular carcinoma (HCC) remain unclear. This study aimed to investigate underlying mecha- nisms of PRIM1 and identify it as a potential molecular target for HCC. Methods: Hub genes were screened between HCC tissues and normal liver tissues in 3 gene expression omnibus (GEO) datasets and the cancer genome atlas (TCGA). The expression features and prognostic value of one of the hub genes PRIM1 were analyzed by bioinformatic analyses and immunohistochemistry. Loss-of-function and gain-of-func- tion studies were used to investigate the regulatory role of PRIM1 in HCC cells. Real-time (RT)-qPCR, western blotting, and ubiquitin immunoprecipitation assays were performed to explore the underlying mechanisms. The xenograft model was employed to detect the roles of PRIM1 in tumor growth in vivo. Finally, the 3D spheroid model was con- ducted to validate the role of PRIM1 in tumor growth and sorafenib resistance. Results: The hub genes of HCC were screened in multiple bioinformatic datasets. PRIM1, as one of the hub genes, was signifcantly overexpressed in HCC tissues in mRNA and protein levels. -

Gene Expression Changes in Cervical Squamous Cancers Following Neoadjuvant Interventional Chemoembolization
Clinica Chimica Acta 493 (2019) 79–86 Contents lists available at ScienceDirect Clinica Chimica Acta journal homepage: www.elsevier.com/locate/cca Gene expression changes in cervical squamous cancers following T neoadjuvant interventional chemoembolization Yonghua Chena,1, Yuanyuan Houa,1, Ying Yanga,1, Meixia Panb, Jing Wanga, Wenshuang Wanga, Ying Zuoa, Jianglin Conga, Xiaojie Wanga, Nan Mua, Chenglin Zhangc, Benjiao Gongc, ⁎ ⁎ Jianqing Houa, , Shaoguang Wanga, , Liping Xud a Department of Obstetrics and Gynecology, the Affiliated Yantai Yuhuangding Hospital of Medical College, Qingdao University, Yantai 264000, Shandong, China b Yantai Yuhuangding Hospital LaiShan Division of Medical College, Qingdao University, China c Central Laboratory, the Affiliated Yantai Yuhuangding Hospital of Medical College, Qingdao University, Yantai 264000, Shandong, China d Medical College, Qingdao University, Qingdao 266021, Shandong, China ARTICLE INFO ABSTRACT Keywords: Background: The efficacy of therapy for cervical cancer is related to the alteration of multiple molecular events Cervical cancer and signaling networks during treatment. The aim of this study was to evaluate gene expression alterations in Chemoembolization advanced cervical cancers before- and after-trans-uterine arterial chemoembolization- (TUACE). Microarray Methods: Gene expression patterns in three squamous cell cervical cancers before- and after-TUACE were de- AKAP12 termined using microarray technique. Changes in AKAP12 and CA9 genes following TUACE were validated by CA9 quantitative real-time PCR. Results: Unsupervised cluster analysis revealed that the after-TUACE samples clustered together, which were separated from the before-TUACE samples. Using a 2-fold threshold, we identified 1131 differentially expressed genes that clearly discriminate after-TUACE tumors from before-TUACE tumors, including 209 up-regulated genes and 922 down-regulated genes. -

Telomere Maintenance Pathway Activity Analysis Enables Tissue- and Gene-Level Inferences
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.01.429081; this version posted February 2, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Telomere maintenance pathway activity analysis enables tissue- and gene-level inferences Lilit Nersisyan1,2*, Arman Simonyan1, Hans Binder3, Arsen Arakelyan1,2 1 Bioinformatics Group, Institute of Molecular Biology, National Academy of Sciences, Yerevan, Armenia 2 Pathverse, LLC, Yerevan, Armenia 3 Interdisciplinary Center for Bioinformatics, University of Leipzig, Leipzig, Germany * Correspondence: Lilit Nersisyan Keywords: telomere maintenance mechanisms, telomerase, alternative lengthening of telomeres, pathway signal flow, testis ABSTRACT Telomere maintenance is one of the mechanisms ensuring indefinite divisions of cancer and stem cells. Good understanding of telomere maintenance mechanisms (TMM) is important for studying cancers and designing therapies. However, molecular factors triggering selective activation of either the telomerase dependent (TEL) or the alternative lengthening of telomeres (ALT) pathway are poorly understood. In addition, more accurate and easy-to-use methodologies are required for TMM phenotyping. In this study, we have performed literature based reconstruction of signaling pathways for the ALT and TEL TMMs. Gene expression data were used for computational assessment of TMM pathway activities and compared with experimental assays for TEL and ALT. Explicit consideration of pathway topology makes bioinformatics analysis more informative compared to computational methods based on simple summary measures of gene expression. Application to healthy human tissues showed high ALT and TEL pathway activities in testis, and identified genes and pathways that may trigger TMM activation.