Telomere Maintenance Pathway Activity Analysis Enables Tissue- and Gene-Level Inferences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How Shelterin Protects Mammalian Telomeres 303 ANRV361-GE42-15 ARI 3 October 2008 10:10 (See 3' 5' TRF2 S Mplex
ANRV361-GE42-15 ARI 3 October 2008 10:10 ANNUAL How Shelterin Protects REVIEWS Further Click here for quick links to Annual Reviews content online, Mammalian Telomeres including: • Other articles in this volume 1 • Top cited articles Wilhelm Palm and Titia de Lange • Top downloaded articles • Our comprehensive search Laboratory for Cell Biology and Genetics, The Rockefeller University, New York, NY 10021; email: [email protected] 1Current address: Max Planck Institute of Molecular Cell Biology and Genetics, 01307 Dresden, Germany Annu. Rev. Genet. 2008. 42:301–34 Key Words First published online as a Review in Advance on ATM, ATR, cancer, NHEJ, HR August 4, 2008 by Rockefeller University on 10/13/09. For personal use only. The Annual Review of Genetics is online at Abstract genet.annualreviews.org The genomes of prokaryotes and eukaryotic organelles are usually cir- This article’s doi: cular as are most plasmids and viral genomes. In contrast, the nuclear Annu. Rev. Genet. 2008.42:301-334. Downloaded from arjournals.annualreviews.org 10.1146/annurev.genet.41.110306.130350 genomes of eukaryotes are organized on linear chromosomes, which Copyright c 2008 by Annual Reviews. require mechanisms to protect and replicate DNA ends. Eukaryotes All rights reserved navigate these problemswith the advent of telomeres, protective nucle- 0066-4197/08/1201-0301$20.00 oprotein complexes at the ends of linear chromosomes, and telomerase, the enzyme that maintains the DNA in these structures. Mammalian telomeres contain a specific protein complex, shelterin, that functions to protect chromosome ends from all aspects of the DNA damage re- sponse and regulates telomere maintenance by telomerase. -

Ailanthone Inhibits Non-Small Cell Lung Cancer Cell Growth Through Repressing DNA Replication Via Downregulating RPA1
FULL PAPER British Journal of Cancer (2017) 117, 1621–1630 | doi: 10.1038/bjc.2017.319 Keywords: ailanthone; non-small cell lung cancer; DNA replication; RPA1; Chinese medicine Ailanthone inhibits non-small cell lung cancer cell growth through repressing DNA replication via downregulating RPA1 Zhongya Ni1, Chao Yao1, Xiaowen Zhu1, Chenyuan Gong1, Zihang Xu2, Lixin Wang3, Suyun Li4, Chunpu Zou2 and Shiguo Zhu*,1,3 1Laboratory of Integrative Medicine, School of Basic Medical Sciences, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Rd, Shanghai 201203, PR China; 2Department of Internal Classic of Medicine, School of Basic Medical Sciences, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Rd, Shanghai 201203, PR China; 3Department of Immunology and Pathogenic Biology, School of Basic Medical Sciences, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Rd, Shanghai 201203, PR China and 4Department of Pathology, School of Basic Medical Sciences, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Rd, Shanghai 201203, PR China Background: The identification of bioactive compounds from Chinese medicine plays a crucial role in the development of novel reagents against non-small cell lung cancer (NSCLC). Methods: High throughput screening assay and analyses of cell growth, cell cycle, apoptosis, cDNA microarray, BrdU incorporation and gene expression were performed. Results: Ailanthone (Aila) suppressed NSCLC cell growth and colony formation in vitro and inhibited NSCLC tumour growth in subcutaneously xenografted and orthotopic lung tumour models, leading to prolonged survival of tumour-bearing mice. Moreover, Aila induced cell cycle arrest in a dose-independent manner but did not induce apoptosis in all NSCLC cells. -

Iron Depletion Reduces Abce1 Transcripts While Inducing The
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 22 October 2019 doi:10.20944/preprints201910.0252.v1 1 Research Article 2 Iron depletion Reduces Abce1 Transcripts While 3 Inducing the Mitophagy Factors Pink1 and Parkin 4 Jana Key 1,2, Nesli Ece Sen 1, Aleksandar Arsovic 1, Stella Krämer 1, Robert Hülse 1, Suzana 5 Gispert-Sanchez 1 and Georg Auburger 1,* 6 1 Experimental Neurology, Goethe University Medical School, 60590 Frankfurt am Main; 7 2 Faculty of Biosciences, Goethe-University Frankfurt am Main, Germany 8 * Correspondence: [email protected] 9 10 Abstract: Lifespan extension was recently achieved in Caenorhabditis elegans nematodes by 11 mitochondrial stress and mitophagy, triggered via iron depletion. Conversely in man, deficient 12 mitophagy due to Pink1/Parkin mutations triggers iron accumulation in patient brain and limits 13 survival. We now aimed to identify murine fibroblast factors, which adapt their mRNA expression 14 to acute iron manipulation, relate to mitochondrial dysfunction and may influence survival. After 15 iron depletion, expression of the plasma membrane receptor Tfrc with its activator Ireb2, the 16 mitochondrial membrane transporter Abcb10, the heme-release factor Pgrmc1, the heme- 17 degradation enzyme Hmox1, the heme-binding cholesterol metabolizer Cyp46a1, as well as the 18 mitophagy regulators Pink1 and Parkin showed a negative correlation to iron levels. After iron 19 overload, these factors did not change expression. Conversely, a positive correlation of mRNA levels 20 with both conditions of iron availability was observed for the endosomal factors Slc11a2 and Steap2, 21 as well as for the iron-sulfur-cluster (ISC)-containing factors Ppat, Bdh2 and Nthl1. -
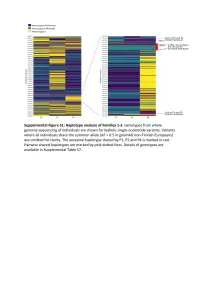
Supplementary Text and Figures.Pdf
Supplemental Figure S2. PRIM1 immunoblots (20-75 kDa). (A) Corresponds to Figure 2D, with additional bands seen in P2 at 75 kDa and 25 kDa. (B) Independent experiment with cell lysates from C and P2 in which additional bands at 25 kDa not evident. (C) Corresponds to Figure 2D. No additional band visible at 25 kDa. (D) Validation of monoclonal antibody (8G10) with siRNA to PRIM1 in HeLa cells. siLUC, luciferase negative control. siPRIM1, siRNA targeting PRIM1 transcript. Vinculin used as a loading control. Supplemental Figure S3: Predicted destabilisation of PRIM1 by the C301R substitution. (A) Cysteine 301 lies in a buried hydrophobic region in PRIM1. DNA primase dimer crystal structure (PDB: 4BPU). PRIM1 residues shaded according to solvent accessibility. Cysteine 301, substituted to arginine in P5, lies in a buried hydrophobic region. (B) Cys301 is evolutionarily conserved in vertebrates. Neither arginine or other large or charged amino acids are observed at this position in other species. (C) The C301R substitution observed in P5 is predicted to lead to destabilisation of all available PRIM1 crystal structures. In contrast, substitution with leucine or threonine, as observed in some orthologous proteins, is not predicted to lead to destabilisation. Data points, predicted changes in free-energy (ΔΔG) from FoldX plotted for each of 14 available crystal structures. (D) Immunoblotting of RPE1 cells transfected with dual reporter constructs (as described in Figure 3B and Materials and Methods) confirms production of PRIM1-GFP and FLAG-SR at expected molecular weights and shows reduced protein levels for the C301R variant. n=2 experiments shown. C301R protein levels relative to WT after normalization to actin loading control indicated underneath the blot. -

4-6 Weeks Old Female C57BL/6 Mice Obtained from Jackson Labs Were Used for Cell Isolation
Methods Mice: 4-6 weeks old female C57BL/6 mice obtained from Jackson labs were used for cell isolation. Female Foxp3-IRES-GFP reporter mice (1), backcrossed to B6/C57 background for 10 generations, were used for the isolation of naïve CD4 and naïve CD8 cells for the RNAseq experiments. The mice were housed in pathogen-free animal facility in the La Jolla Institute for Allergy and Immunology and were used according to protocols approved by the Institutional Animal Care and use Committee. Preparation of cells: Subsets of thymocytes were isolated by cell sorting as previously described (2), after cell surface staining using CD4 (GK1.5), CD8 (53-6.7), CD3ε (145- 2C11), CD24 (M1/69) (all from Biolegend). DP cells: CD4+CD8 int/hi; CD4 SP cells: CD4CD3 hi, CD24 int/lo; CD8 SP cells: CD8 int/hi CD4 CD3 hi, CD24 int/lo (Fig S2). Peripheral subsets were isolated after pooling spleen and lymph nodes. T cells were enriched by negative isolation using Dynabeads (Dynabeads untouched mouse T cells, 11413D, Invitrogen). After surface staining for CD4 (GK1.5), CD8 (53-6.7), CD62L (MEL-14), CD25 (PC61) and CD44 (IM7), naïve CD4+CD62L hiCD25-CD44lo and naïve CD8+CD62L hiCD25-CD44lo were obtained by sorting (BD FACS Aria). Additionally, for the RNAseq experiments, CD4 and CD8 naïve cells were isolated by sorting T cells from the Foxp3- IRES-GFP mice: CD4+CD62LhiCD25–CD44lo GFP(FOXP3)– and CD8+CD62LhiCD25– CD44lo GFP(FOXP3)– (antibodies were from Biolegend). In some cases, naïve CD4 cells were cultured in vitro under Th1 or Th2 polarizing conditions (3, 4). -
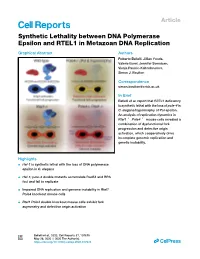
Synthetic Lethality Between DNA Polymerase Epsilon and RTEL1 in Metazoan DNA Replication
Article Synthetic Lethality between DNA Polymerase Epsilon and RTEL1 in Metazoan DNA Replication Graphical Abstract Authors Roberto Bellelli, Jillian Youds, Valerie Borel, Jennifer Svendsen, Visnja Pavicic-Kaltenbrunner, Simon J. Boulton Correspondence [email protected] In Brief Bellelli et al. report that RTEL1 deficiency is synthetic lethal with the loss of pole-4 in C. elegans/hypomorphy of Pol epsilon. An analysis of replication dynamics in Rtel1À/À Pole4À/À mouse cells revealed a combination of dysfunctional fork progression and defective origin activation, which cooperatively drive incomplete genomic replication and genetic instability. Highlights d rtel-1 is synthetic lethal with the loss of DNA polymerase epsilon in C. elegans d rtel-1; pole-4 double mutants accumulate Rad51 and RPA foci and fail to replicate d Impaired DNA replication and genome instability in Rtel1 Pole4 knockout mouse cells d Rtel1 Pole4 double knockout mouse cells exhibit fork asymmetry and defective origin activation Bellelli et al., 2020, Cell Reports 31, 107675 May 26, 2020 ª 2020 The Author(s). https://doi.org/10.1016/j.celrep.2020.107675 ll ll OPEN ACCESS Article Synthetic Lethality between DNA Polymerase Epsilon and RTEL1 in Metazoan DNA Replication Roberto Bellelli,1,2,3 Jillian Youds,1,2 Valerie Borel,1 Jennifer Svendsen,1 Visnja Pavicic-Kaltenbrunner,1 and Simon J. Boulton1,4,* 1The Francis Crick Institute, 1 Midland Road, NW1 1AT London, UK 2These authors contributed equally 3Present address: Center for Cancer Cell and Molecular Biology, Barts Cancer Institute, Queen Mary University of London, Charterhouse Square, Barbican, EC1M 6BE London, UK 4Lead Contact *Correspondence: [email protected] https://doi.org/10.1016/j.celrep.2020.107675 SUMMARY Genome stability requires coordination of DNA replication origin activation and replication fork progression. -

Dyskerin Mutations Present in Dyskeratosis Congenita Patients Increase Oxidative Stress and DNA Damage Signalling in Dictyostelium Discoideum
cells Article Dyskerin Mutations Present in Dyskeratosis Congenita Patients Increase Oxidative Stress and DNA Damage Signalling in Dictyostelium Discoideum Javier Rodriguez-Centeno, Rosario Perona and Leandro Sastre * Instituto de Investigaciones Biomedicas, CSIC/UAM and Centro de Investigación Biomédica en Red de Enfermedades Raras, CIBERER, 28029 Madrid, Spain; [email protected] (J.R.-C.); [email protected] (R.P.) * Correspondence: [email protected]; Tel.: +3491-585-4437 Received: 11 October 2019; Accepted: 5 November 2019; Published: 8 November 2019 Abstract: Dyskerin is a protein involved in the formation of small nucleolar and small Cajal body ribonucleoproteins. These complexes participate in RNA pseudouridylation and are also components of the telomerase complex required for telomere elongation. Dyskerin mutations cause a rare disease, X-linked dyskeratosis congenita, with no curative treatment. The social amoeba Dictyostelium discoideum contains a gene coding for a dyskerin homologous protein. In this article D. discoideum mutant strains that have mutations corresponding to mutations found in dyskeratosis congenita patients are described. The phenotype of the mutant strains has been studied and no alterations were observed in pseudouridylation activity and telomere structure. Mutant strains showed increased proliferation on liquid culture but reduced growth feeding on bacteria. The results obtained indicated the existence of increased DNA damage response and reactive oxygen species, as also reported in human Dyskeratosis congenita cells and some other disease models. These data, together with the haploid character of D. discoideum vegetative cells, that resemble the genomic structure of the human dyskerin gene, located in the X chromosome, support the conclusion that D. discoideum can be a good model system for the study of this disease. -

POLE2 Knockdown Reduce Tumorigenesis in Esophageal Squamous Cells Yongjun Zhu, Gang Chen, Yang Song, Zhiming Chen* and Xiaofeng Chen*
Zhu et al. Cancer Cell Int (2020) 20:388 https://doi.org/10.1186/s12935-020-01477-4 Cancer Cell International PRIMARY RESEARCH Open Access POLE2 knockdown reduce tumorigenesis in esophageal squamous cells Yongjun Zhu, Gang Chen, Yang Song, Zhiming Chen* and Xiaofeng Chen* Abstract Background: Esophageal squamous cell carcinoma (ESCC) is one of the most frequent malignant tumors originated from digestive system around the world and the treatment was limited by the unclear mechanism. DNA polymerase epsilon 2, accessory subunit (POLE2) is involved in DNA replication, repair, and cell cycle control, whose association with ESCC is still not clear. Methods: In this study, the expression level of POLE2 in ESCC tissues was detected by IHC. The POLE2 knockdown cell line was constructed, identifed by qPCR and western blot and used for detecting cellular functions and con- structing xenotransplantation mice model. MTT Assay, colony formation assay, fow cytometry, wound-healing assay and Transwell assay were used to detected cell proliferation, apoptosis and migration. Results: We frstly identifed that the expression of POLE2 was overexpressed in ESCC. Moreover, the high expres- sion of POLE2 can predict the tumor deterioration and poor prognosis of ESCC patients. Additionally, downregulation of POLE2 was involved in ESCC progression by promoting proliferation, migration, and inhibiting apoptosis in vitro. In vivo studies proved that POLE2 was positively correlated with ESCC tumor formation, which was consistent with the results in vitro. We also illuminated that POLE2 knockdown upregulated pro-apoptotic proteins (Bax, Caspase3, CD40L, FasL, IGFBP-5 and P21) and downregulated anti-apoptotic proteins (CLAP-2, IGF-I and sTNF-R2). -

Structure of the Human Clamp Loader Reveals an Autoinhibited Conformation of a Substrate-Bound AAA+ Switch
Structure of the human clamp loader reveals an autoinhibited conformation of a substrate-bound AAA+ switch Christl Gaubitza,1, Xingchen Liua,b,1, Joseph Magrinoa,b, Nicholas P. Stonea, Jacob Landecka,b, Mark Hedglinc, and Brian A. Kelcha,2 aDepartment of Biochemistry and Molecular Pharmacology, University of Massachusetts Medical School, Worcester MA 01605; bGraduate School of Biomedical Sciences, University of Massachusetts Medical School, Worcester MA 01605; and cDepartment of Chemistry, The Pennsylvania State University, University Park, PA 16802 Edited by Michael E. O’Donnell, HHMI and Rockefeller University, New York, NY, and approved July 27, 2020 (received for review April 20, 2020) DNA replication requires the sliding clamp, a ring-shaped protein areflexia syndrome (15), Hutchinson–Gilford progeria syn- complex that encircles DNA, where it acts as an essential cofactor drome (16), and in the replication of some viruses (17–19). It for DNA polymerases and other proteins. The sliding clamp needs is unknown whether loading by RFC contributes to PARD to be opened and installed onto DNA by a clamp loader ATPase of disease. the AAA+ family. The human clamp loader replication factor C Clamp loaders are members of the AAA+ family of ATPases (RFC) and sliding clamp proliferating cell nuclear antigen (PCNA) (ATPases associated with various cellular activities), a large are both essential and play critical roles in several diseases. De- protein family that uses the chemical energy of adenosine 5′- spite decades of study, no structure of human RFC has been re- triphosphate (ATP) to generate mechanical force (20). Most solved. Here, we report the structure of human RFC bound to AAA+ proteins form hexameric motors that use an undulating PCNA by cryogenic electron microscopy to an overall resolution ∼ spiral staircase mechanism to processively translocate a substrate of 3.4 Å. -
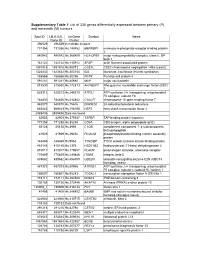
Supplementary Table 1 List of 335 Genes Differentially Expressed Between Primary (P) and Metastatic (M) Tumours
Supplementary Table 1 List of 335 genes differentially expressed between primary (P) and metastatic (M) tumours Spot ID I.M.A.G.E. UniGene Symbol Name Clone ID Cluster 296529 296529 In multiple clusters 731356 731356 Hs.140452 M6PRBP1 mannose-6-phosphate receptor binding protein 1 840942 840942 Hs.368409 HLA-DPB1 major histocompatibility complex, class II, DP beta 1 142122 142122 Hs.115912 AFAP actin filament associated protein 1891918 1891918 Hs.90073 CSE1L CSE1 chromosome segregation 1-like (yeast) 1323432 1323432 Hs.303154 IDS iduronate 2-sulfatase (Hunter syndrome) 788566 788566 Hs.80296 PCP4 Purkinje cell protein 4 591281 591281 Hs.80680 MVP major vault protein 815530 815530 Hs.172813 ARHGEF7 Rho guanine nucleotide exchange factor (GEF) 7 825312 825312 Hs.246310 ATP5J ATP synthase, H+ transporting, mitochondrial F0 complex, subunit F6 784830 784830 Hs.412842 C10orf7 chromosome 10 open reading frame 7 840878 840878 Hs.75616 DHCR24 24-dehydrocholesterol reductase 669443 669443 Hs.158195 HSF2 heat shock transcription factor 2 2485436 2485436 Data not found 82903 82903 Hs.370937 TAPBP TAP binding protein (tapasin) 771258 771258 Hs.85258 CD8A CD8 antigen, alpha polypeptide (p32) 85128 85128 Hs.8986 C1QB complement component 1, q subcomponent, beta polypeptide 41929 41929 Hs.39252 PICALM phosphatidylinositol binding clathrin assembly protein 148469 148469 Hs.9963 TYROBP TYRO protein tyrosine kinase binding protein 415145 415145 Hs.1376 HSD11B2 hydroxysteroid (11-beta) dehydrogenase 2 810017 810017 Hs.179657 PLAUR plasminogen activator, -

Gene Expression Profiling Analysis Contributes to Understanding the Association Between Non-Syndromic Cleft Lip and Palate, and Cancer
2110 MOLECULAR MEDICINE REPORTS 13: 2110-2116, 2016 Gene expression profiling analysis contributes to understanding the association between non-syndromic cleft lip and palate, and cancer HONGYI WANG, TAO QIU, JIE SHI, JIULONG LIANG, YANG WANG, LIANGLIANG QUAN, YU ZHANG, QIAN ZHANG and KAI TAO Department of Plastic Surgery, General Hospital of Shenyang Military Area Command, PLA, Shenyang, Liaoning 110016, P.R. China Received March 10, 2015; Accepted December 18, 2015 DOI: 10.3892/mmr.2016.4802 Abstract. The present study aimed to investigate the for NSCL/P were implicated predominantly in the TGF-β molecular mechanisms underlying non-syndromic cleft lip, signaling pathway, the cell cycle and in viral carcinogenesis. with or without cleft palate (NSCL/P), and the association The TP53, CDK1, SMAD3, PIK3R1 and CASP3 genes were between this disease and cancer. The GSE42589 data set found to be associated, not only with NSCL/P, but also with was downloaded from the Gene Expression Omnibus data- cancer. These results may contribute to a better understanding base, and contained seven dental pulp stem cell samples of the molecular mechanisms of NSCL/P. from children with NSCL/P in the exfoliation period, and six controls. Differentially expressed genes (DEGs) were Introduction screened using the RankProd method, and their potential functions were revealed by pathway enrichment analysis and Non-syndromic cleft lip, with or without cleft palate (NSCL/P) construction of a pathway interaction network. Subsequently, is one of the most common types of congenital defect and cancer genes were obtained from six cancer databases, and affects 3.4-22.9/10,000 individuals worldwide (1). -

Polymerase Δ Deficiency Causes Syndromic Immunodeficiency with Replicative Stress
Polymerase δ deficiency causes syndromic immunodeficiency with replicative stress Cecilia Domínguez Conde, … , Mirjam van der Burg, Kaan Boztug J Clin Invest. 2019. https://doi.org/10.1172/JCI128903. Research Article Genetics Immunology Graphical abstract Find the latest version: https://jci.me/128903/pdf The Journal of Clinical Investigation RESEARCH ARTICLE Polymerase δ deficiency causes syndromic immunodeficiency with replicative stress Cecilia Domínguez Conde,1,2 Özlem Yüce Petronczki,1,2,3 Safa Baris,4,5 Katharina L. Willmann,1,2 Enrico Girardi,2 Elisabeth Salzer,1,2,3,6 Stefan Weitzer,7 Rico Chandra Ardy,1,2,3 Ana Krolo,1,2,3 Hanna Ijspeert,8 Ayca Kiykim,4,5 Elif Karakoc-Aydiner,4,5 Elisabeth Förster-Waldl,9 Leo Kager,6 Winfried F. Pickl,10 Giulio Superti-Furga,2,11 Javier Martínez,7 Joanna I. Loizou,2 Ahmet Ozen,4,5 Mirjam van der Burg,8 and Kaan Boztug1,2,3,6 1Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases, 2CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, and 3St. Anna Children’s Cancer Research Institute (CCRI), Vienna, Austria. 4Pediatric Allergy and Immunology, Marmara University, Faculty of Medicine, Istanbul, Turkey. 5Jeffrey Modell Diagnostic Center for Primary Immunodeficiency Diseases, Marmara University, Istanbul, Turkey. 6St. Anna Children’s Hospital, Department of Pediatrics and Adolescent Medicine, Vienna, Austria. 7Center for Medical Biochemistry, Medical University of Vienna, Vienna, Austria. 8Department of Pediatrics, Laboratory for Immunology, Leiden University Medical Centre, Leiden, Netherlands. 9Department of Neonatology, Pediatric Intensive Care and Neuropediatrics, Department of Pediatrics and Adolescent Medicine, 10Institute of Immunology, Center for Pathophysiology, Infectiology and Immunology, and 11Center for Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria.