NMR-Based Metabolomics in Wine Quality Control and Authentication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Artemis Karamolegos Wines Descriptions for Wine Experts
ARTEMIS KARAMOLEGOS WINES DESCRIPTIONS FOR WINE EXPERTS Having its roots in the volcanic soil of Santorini and tradition that goes back to 1952, the winery of Artemis Karamolegos is one of the most dynamic and rapidly evolving wineries of Santorini. All its wines have been distinguished in several important International Wine Competitions. The winery has the most modern facilities for wine production, spaces for wine testing and a shop for wines and selected local products. Artemis Karamolegos had the innovative idea to combine the experience of a tour at the winery with lunch at the restaurant Aroma Avlis, the menu of which has the signature of the talented chef Christos Coskinas. In its spacious new yard offering view to the vineyard and the beaches of Monolithos and Avis, as well as in the dinning halls, you can taste the delicious Mediterranean and local dishes made with fresh, carefully selected local products, accompanied with wines from the winery. The history of the winery goes back to the 1952, where the grandfather, Artemis, was cultivating the vineyards in order to produce wine for his own family and later on, in order to sell it in the island and in the rest of Greece. Artemis Karamolegos, the grandson who succeeded his grandfather and his father at the winery of Exo Gonia, is an energetic young man full of passion for his Job. Since 2004 and until today, he managed to lead, miraculously, the family business many steps ahead very fast. In 2004, a turn to a modern and of a high quality production winery took place, with the production of a bottled, labeled and of a good quality wine named “SANTORINI”. -
Wine Map of the Peleponnese 2014
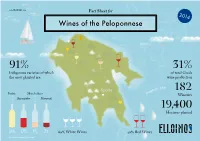
www.ELLOINOS.com Fact Sheet for 2014 Wines of the Peloponnese Patras Athens 91% 31% Indigenous varieties of which of total Greek the most planted are: wine production n Sea Sparta gea 182 Ae Roditis Moschofilero Wineries Agiorgitiko Mavroudi 19,400 Hectares planted 34% 17% 9% 7% 60% White Wines 40% Red Wines Information design by ideologio Protected Designation of Origin Wine Colors Muscat of Rio Patras Grape: Muscat Blanc Mavrodaphne of Patras Grapes: Mavrodaphne, Korinthiaki Athens Nemea Grape Agiorgitiko Muscat of Patras Mantinia Grape: Muscat Blanc Grape Moschofilero Patras Epidaurus Grape: Roditis Sea ean eg Kalamata A In the EU, schemes of geographical indications known as Protected Designation of Origin (PDO) and Protected Monemvassia Geographical Indication (PGI), promote and protect names of —Malvasia quality agricultural and food products. Amongst many other products, the names of wines are also protected by these Grapes: Monemvassia (min 51%), laws. Assyrtiko, Asproudes, Kydonitsa PDO products are prepared, processed, and produced in a given geographical area, using recognized know-how and therefore acquire unique properties. White Wine Sweet White Wine Red Wine Sweet Red Wine Indigenous grapes International grapes Region Note There are additional grape varieties allowed, but PGI products are closely linked to the geographical current plantings are small. area in which they are traditionally and at least White indigenous: Asproudes Patras, Aidani, partially manufactured (prepared, processed OR Assyrtiko, Athiri, Glikerithra, Goustolidi, Laghorthi, produced), and have specific qualities attributable to Migdali, Petroulianos, Potamissi, Robola, Rokaniaris, Skiadopoulo, Sklava, Volitsa Aspro. that geographical area, therefore acquiring unique properties. Depending on their geographical breadth, Red indigenous: Limniona, Skylopnichtis, Thrapsa, Voidomatis, Volitsa. -
Table of Contents
TABLE OF CONTENTS Sparkling & Champagne ............................. 3 White Wine .................................................. 4 Greece ........................................................................................4 Mediterranean ......................................................................5 Germany ...................................................................................5 Italy ................................................................................................5 Spain ........................................................................................... 6 France ........................................................................................ 6 From the New World .......................................................7 Rosé Wine ................................................ 8 Skin-Contact Wine ................................... 9 Red Wine .................................................10 Greece .............................................................................10 Mediterranean ...........................................................12 Italy ..................................................................................... 12 Spain .................................................................................. 13 France............................................................................... 13 From the New World ............................................ 14 Thrace Macedonia Epirius Thessaly Ionian Islands Aegean Peloponnese Islands Crete 2 SPARKLING -

WINES SELECTION Italian Law Quality Guarantee Marks
DRINK LIST OUR BEST WINES SELECTION Italian Law Quality Guarantee Marks D.O.C. Controlled Origin D.O.C.G. Controlled and Guaranteed Origin GREEK WINES WHITE WINES ESTATE P.D.O. OAK BARELS MORAITIS Winery: Moraitis Features: Dry white wine, 80% of the wine is fermented in a stainless steel vat with 6 months maturation on its lees. The rest Area of Origin: Paros , Greece 20% is fermented and matured for 6 months in new French oak barrels. Bright yellow gold color. Intense aromas of exotic fruits Grape Variety: 100% Monemvassia/ and citrus. The oak is well integrated and the crispness and elegan Vineyard Kamares ce of the wine easily dominates. Alcohol: 13 %vol. Price per Bottle 38 T S E KTIMA ALFA B E C C H OI Winery: Alpha Estate Area of Origin: Amyntaion, Greece Features: Bright white wine with intense flavor and rich aroma of citrus. Grape Variety: Sauvignon Blanc 100% Price per Bottle 29,5 Alcohol: 13,5 %vol. ESTATE MALAGOUSIA BIO MORAITIS Winery: Moraitis Features: Dry white wine, pale white yellow colour with green Area of Origin: Paros , Greece reflections. Rich in citrus and exotic fruit aromas. On the palate it is balanced, fruity, with notes of lemon peel and grapefruit. Grape Variety: Malagousia Price per Bottle 28,5 Alcohol: 12,5 %vol. CHARDONNAY GEROVASSILIU Winery: Gerovassiliu Area of Origin: Epanomi-Salonicco, Features: White wine aged in oak barrels, with a strong flavor and Greece characteristic with notes of citrus, dried fruit and vanilla. Grape Variety: Chardonnay Price per Bottle 33,5 Alcohol: 12,5 %vol. -

Phenolic Compounds As Markers of Wine Quality and Authenticity
foods Review Phenolic Compounds as Markers of Wine Quality and Authenticity Vakare˙ Merkyte˙ 1,2 , Edoardo Longo 1,2,* , Giulia Windisch 1,2 and Emanuele Boselli 1,2 1 Faculty of Science and Technology, Free University of Bozen-Bolzano, Piazza Università 5, 39100 Bozen-Bolzano, Italy; [email protected] (V.M.); [email protected] (G.W.); [email protected] (E.B.) 2 Oenolab, NOI Techpark South Tyrol, Via A. Volta 13B, 39100 Bozen-Bolzano, Italy * Correspondence: [email protected]; Tel.: +39-0471-017691 Received: 29 October 2020; Accepted: 28 November 2020; Published: 1 December 2020 Abstract: Targeted and untargeted determinations are being currently applied to different classes of natural phenolics to develop an integrated approach aimed at ensuring compliance to regulatory prescriptions related to specific quality parameters of wine production. The regulations are particularly severe for wine and include various aspects of the viticulture practices and winemaking techniques. Nevertheless, the use of phenolic profiles for quality control is still fragmented and incomplete, even if they are a promising tool for quality evaluation. Only a few methods have been already validated and widely applied, and an integrated approach is in fact still missing because of the complex dependence of the chemical profile of wine on many viticultural and enological factors, which have not been clarified yet. For example, there is a lack of studies about the phenolic composition in relation to the wine authenticity of white and especially rosé wines. This review is a bibliographic account on the approaches based on phenolic species that have been developed for the evaluation of wine quality and frauds, from the grape varieties (of V. -

University of Cincinnati
UNIVERSITY OF CINCINNATI Date:___________________ I, _________________________________________________________, hereby submit this work as part of the requirements for the degree of: in: It is entitled: This work and its defense approved by: Chair: _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ The Role of Wine Production in the Changing Structure of an Island Economy: A Case Study of Santorini Greece A thesis submitted to Division of Research and Advanced Studies of the University of Cincinnati in partial fulfillment of the requirements for the degree of MASTER OF COMMUNITY PLANNING School of Planning College of Design, Architecture, Art, and Planning May 13, 2005 By Curt Christopher Freese B.A. History, University of Cincinnati, 2001 Thesis Committee Chair: Michael Romanos, Ph.D. Member: Carla Chifos, Ph. D. Reader: Wayne Durrill, Ph. D. History Abstract: The inspiration for this thesis arose out of a summer spent in 2004 on the island of Santorini as part of the University of Cincinnati’s Sustainable Development Program. While on this enchanting island, this author couldn’t help but notice the oddly beautiful vineyards that were growing in soil that looked no different from the surface of the moon, nor indulge in a few glasses of wine produced from grapes grown under the incessant pounding heat of a hundred-degree sun. Much to my surprise, the wine had a most fascinating taste unlike anything I had never experienced before. Yet, as the days passed on the island, and I took in more sights of its interesting vineyards on terraces or on hills, and I experienced more of its wine. I became quite shocked with what I witnessed and tasted. -

Vaso Wine List Final 2016-01-17
WINE LIST RED WINE Glass Bottle Greek Red Wines Limnio 2012 – Limnio "Organic" (Ktima Vourvoukeli Winery, Avdira, Greece) 12 36 Paros Moraitis Reserve 2009 – Mandilaria & Monemvassia (Paros, Greece) 13 39 Alexandra's Nostos 2012 – Syrah, Grenache, Mourvedre (Vatolakkos, Crete) 14 42 Red by JK 2011 – Cabernet , Merlot, Xynomavro (Mount Velventos, Greece) 12 38 Naoussea 2008 – Xynomavro (Naoussa, Greece) 12 36 Nemea 2010 – Agiorgitiko (Nemea, Greece) 12 36 King Of Hearts 2013 – Cabernet, Merlot (Nico Lazaridis Chateau, Drama, Greece) 11 36 The Black SheeP 2013 – Syrah, Merlot (Nico Lazaridis Chateau, Mount Pangeon, Greece) 11 36 Award Winning Greek Red Wines Amethystos 2011 – Cabernet , Merlot, Agiorgitiko (Lazardis Vineyards, Drama, Greece) - 49 Areti 2008 – Agiorgitko (Biblia Chora, Kokkinochori, Kavala, Greece) - 71 Magic Mountain (Μαγικό Βουνό) 2009 – Cabernet Sauv, Cabernet Franc (Drama, Greece) - 79 Avantis Estate 2011 – Mandilaria (Evia Island, Greece) - 51 Perpetuus 2007 – Sangiovese, Cabernet Sauv (Drama, Greece) - 81 Heritage 2012 – Maratheftiko (Keo Winery, Pitsillia, Cyprus) - 51 Italian Red Wines Rocche Costamagna Barbera D'Alba 2013 – Nebbiolo, Barbera (Piedmont, Italy) 13 39 San Vito Chianti 2013 – Sangiovese (Florence, Tuscany, Italy) 10 31 ValPolicella Classico SuPeriore 2012 – RiPasso (Verona, Italy) 12 36 Brancaia Chianti Classico Riserva 2011 – Sangiovese (DOCG, Tuscany, Italy) - 78 Ca’Bea del Maniero 2012 – Pinot Noir (Pavia, Italy) 11 33 Poggio al Tesoro Sondraia 2011 – Cabernet Sauv, Merlot, Cabernet Franc (Tuscany, -

Curriculum Studies Guide
TECHNOLOGICAL EDUCATIONAL INSTITUTE OF ATHENS FACULTY OF FOOD TECHNOLOGY & NUTRITION DEPARTMENT OF OENOLOGY & BEVERAGE TECHNOLOGY CURRICULUM STUDIES GUIDE ACADEMIC YEAR 2010-2011 CURRICULUM FOR THE ACADEMIC YEAR 2010-2011 Contact Details: Address St. Spyridonos, 12210, Aigaleo Head of the Department Panagiotis Kaldis, Professor 210-5385503 Department Secretariat Eleni Zoulinou, Chief Secretary: Chrysso Bogiatzi 210-5385538 210-5385504 Fax 210-5385504 Department Secretariat Email [email protected] Department Email [email protected] Department Website www.teiath.gr/stetrod/oenology T.E.I. of Athens Website www.teiath.gr Curriculum Studies Guide Text: Elias Korkas, Assistant Professor Curriculum Studies Guide Editing: Chrysso Bogiatzi, Department Secretariat CURRICULUM FOR THE ACADEMIC YEAR 2010-2011 CONTENTS 1 HISTORY OF THE DEPARTMENT 1 2 CONTENT OF THE DEPARTMENT’S STUDIES 5 3 ORGANIZATION – ADMINISTRATION OF THE DEPARTMENT 12 4 DEPARTMENT STAFF 14 5 DEPARTMENT FACILITIES 16 6 RESEARCH ACTIVITIES – SEMINARS - WORKSHOPS 17 7 CURRICULUM 18 8 PRACTICAL TRAINING GUIDE 137 9 DISSERTATION GUIDE 139 CURRICULUM FOR THE ACADEMIC YEAR 2010-2011 TECHNOLOGICAL FACULTY OF FOOD TECHNOLOGY & NUTRITION EDUCATIONAL DEPARTMENT OF OENOLOGY & BEVERAGE TECHNOLOGY INSTITUTE OF ATHENS 1 HISTORY OF THE DEPARTMENT In Greece the provision of a comprehensive higher education course exclusively devoted to oenology has been a standing request of the wine industry since 1983, when the Technological Educational Institutes were first established. At that time a few courses in oenology or viticulture were taught at University Agricultural Faculties, in the Faculties of Food Technology of the TEI Institutes in Athens and Thessaloniki (oenology courses only) and at the Agricultural Technology Faculties of the TEI Institutes (viticulture courses only). -

5761 Juliette, Domaine De La Sangliere, Provence, France ’17 $12 Juliette Is Refreshing and Elegant, Bone Dry Rosé Wine, with Layers of Lively Fruit
WINE STORE. WINE DELIVERY. TH 301 5 AVE S NAPLES 239 360 2000 SPECIALIZING IN ORGANIC, SMALL PRODUCTION & HIGHLY ALLOCATED WINES SPARKLING WINE, CHAMPAGNE & ROSE SPECIALS 5761 Juliette, Domaine de la Sangliere, Provence, France ’17 $12 Juliette is refreshing and elegant, bone dry rosé wine, with layers of lively fruit. 1133 Codorniu ‘Dulce Anna’ Cava, Spain ‘NV $12 Fresh and versatile made from Chardonnay (70%) and traditional varieties Macabeo, Xarel·lo and Parellada. Fantastic combination of fruity aromas with sweet balance in the mouth. Fresh notes of fruit and white spring flowers, memories of peaches and almond blossoms. The palate brings a pleasant sensation of density, while maintaining a great freshness and vitality. 1127 Oro Del Doge, Spumante, Italy ’NV $12 The nose is delicate, fresh and fruity scents, reminiscent of golden apples, peaches and acacia flowers. Also fragrant in tasting, characterized by a good freshness and an equally good persistence. 1130 Sassetti Livio Pertimali, Prosecco Superiore Brut, Italy NV $29 Italy’s highest-quality designation in the Prosecco category. Situated 30 miles north of Venice and 63 miles south of the Dolomites in the province of Treviso. Hand harvesting and cultivation occur in the steep hillsides, the birthplace of Prosecco, and while incredibly labor-intensive, also drive quality grape selection and an artisanal approach throughout. 1042 Tenuta Ambrosini Batudé Brut, Franciacorta, Italy NV $35 70% Chardonnay, 20% Pinot Noir, 10% Pinot Blanc. Straw-yellow color with golden reflection, fine and persistent perlage, elegant and delicate flowery scent, balanced and soft. Deep in the heart of Franciacorta, where the Iseo lake meets the scenic, sunbathed Pre-Alps hill the Ambrosini family and their 8 hectare vineyard located in the town of Cazzago S.Martino, one of the 19 towns belonging to the protected and controlled area where Franciacorta wine can be produced. -

O R N G E B U B B L Y R O
All of the wines featured at Committee are made the old-school way: native E grapes, traditional winemaking practices, hands-on in the vineyard and hands- off in the cellar. We’re proud to work with only family-owned estates (and one SIP N amazing cooperative), and we’re even more excited to show you the beauty, depth, I and unique expression of heritage Greek wines. Enjoy a glass of some- thing delicious, or turn the page for bottles of wine from producers honoring thousands of W years of winemaking (and drinking) history in Greece and beyond. RETSINA glass bottle 17 Kechris, ‘Tear of the Pine’ Retsina, Thessaloniki 14 56 18 Kechris, ‘Kechribari’ Retsina, Thessaloniki, 500ml 14 SPARKLING 17 Domaine Karanika ‘Cuvee Speciale,’ Amyndaio 18 72 18 Kir-Yianni Rosé ‘Akakies,’ Amyndaio 13 52 WHITE 18 Moschofilero, Troupis ‘Fteri,’ Arkadia 12 48 18 Assyrtiko, Alexakis, Crete 16 64 18 Assyrtiko / Aidani / Athiri, Argyros ‘Atlantis,’ Cyclades Islands 14 56 18 Malagousia, Domaine Zefeirakis, Tyrnavos 15 60 ORANGE 18 Santameriana, Sant’Or ‘Nature,’ Achaia 16 64 ROSÉ 18 Malagousia, Mylonas, Attiki 12 48 19 Limniona, Domaine Zafeirakis, Trynavos 14 56 RED 15 Xinomavro, Thymiopoulos, Naoussa 15 60 15 Cabernet Sauvignon/Merlot, Tselepos ‘Dilofos,’ Tegea 17 72 15 Agiorgitiko, Ktima ‘Driopi,’ Nemea 15 60 16 Mandilaria, Lyrarakis ‘Plakoura Vineyards,’ Crete 15 60 16 Tsapournakos, Voyatzi, Velvento 16 64 CARAFE white/red καράφα Please ask your server! 32 BY THE BOTTLE Y ί 17 Domaine Spiropoulos ‘Ode Panos’ Brut, Mantinia, Peloponnese 58 σ Stone fruits and flowers. Nectar of the gods. α L ρ κ B ς 13 Tselepos ‘Amalia’ Brut, Nemea, Peloponnese 90 ε δ Better than Veuve. -

Page 1 the I/M List Brings You a Lick of Levantine Wine to Match the Cuisine
RACY + PURE The I/M list brings you a lick of Levantine wine to match the cuisine, along with island 18 Frankland Estate ‘Isolation Ridge’ Riesling wines from around the world, including our own big chunk o’ land, abundant in coastline Great Southern, WA 85 & girt by sea, Tassie included. Think NZ, Sicily, Sardinia, Crete, Corsica, Mallorca... 18 Brand & Brand Riesling KABINETT Pfalz, GER 75 Along with Mediterranean numbers that share the same provenance as our food. There are exceptions, of course; ‘cos sometimes there’s just fun stuff we wanna drink. 20 South by South West Sauv Blanc Margaret River, WA 60 20 Mount Majura Pinot Gris Canberra District, ACT 60 18 Guilhem & J-Huges Goisot Aligoté Burgundy, FRA 75 WHITEBY THE GLASS 150ML 500ML 750ML 18 Menade Verdejo Rueda, ESP 55 19 I/M ‘White Plonk’ Great Southern, WA 9 30 45 16 Envinate ‘Benje’ Listan Blanco Tenerife, ESP 95 18 Hees Riesling TROCKEN Nahe, GER 14 47 70 Horgelus Sauv Blanc Blend 19 Côtes de Gascogne, FRA 13 44 65 LIFTED + FLORAL 19 Piattini Pinot Grigio Veneto, ITA 12 40 60 Fiano 19 Nomads Garden Grüner Veltliner King Valley, VIC 11 37 55 20 Vino Volta ‘So Well Then’ Swan District, WA 70 19 Unico Zelo ‘Slate Farm’ Fiano Clare Valley, SA 75 20 Skigh ‘Abacus’ Chardonnay Margaret River, WA 15 50 75 18 Massena ‘Fruit Salad’ Muscat Blend Eden Valley, SA 55 19 Somos ‘Blanquito’ Arneis/Garganega McLaren Vale, SA 60 WINES WITH FIZZ 125ML 750ML 20 Site Pinot Gris SKINSY King Valley, VIC 60 NV I/M ‘Fizzy Plonk’ Multi Region, VIC 9 52 18 Scout Chardonnay Central Otago, NZ 75 18 Nick -

D8.1 - Piloting Plan
Big Data to Enable Global Disruption of the Grapevine-powered Industries D8.1 - Piloting Plan DELIVERABLE NUMBER D8.1 DELIVERABLE TITLE Piloting Plan RESPONSIBLE AUTHOR Aikaterini Kasimati (AUA) Co-funded by the Horizon 2020 Framework Programme of the European Union Big Data to Enable Global Disruption of the Grapevine-powered industries GRANT AGREEMENT N. 780751 PROJECT ACRONYM BigDataGrapes Big Data to Enable Global Disruption of the Grapevine-powered PROJECT FULL NAME industries STARTING DATE (DUR.) 01/01/2018 (36 months) ENDING DATE 31/12/2020 PROJECT WEBSITE http://www.bigdatagrapes.eu/ COORDINATOR Nikos Manouselis ADDRESS 110 Pentelis Str., Marousi, GR15126, Greece REPLY TO [email protected] PHONE +30 210 6897 905 EU PROJECT OFFICER Ms. Annamária Nagy WORKPACKAGE N. | TITLE WP8 | Grapevine-powered Industry Application Pilots WORKPACKAGE LEADER Agricultural University of Athens DELIVERABLE N. | TITLE D8.1 | Piloting Plan RESPONSIBLE AUTHOR Aikaterini Kasimati (AUA) REPLY TO [email protected] DOCUMENT URL http://www.bigdatagrapes.eu/ 30 June 2018 (M6), 31 March 2019 (M15, updated version), 6 August DATE OF DELIVERY (CONTRACTUAL) 2019 (M20, Updated Version) 29 June 2018 (M6), 29 March 2019 (M15, updated version), 6 DATE OF DELIVERY (SUBMITTED) August 2019 (M20, Updated Version) VERSION | STATUS 3.0 | Final NATURE Report (R) DISSEMINATION LEVEL Public (PU) AUTHORS (PARTNER) Aikaterini Kasimati (AUA) Spyros Fountas (AUA), Evangelos Anastasiou (AUA), Maritina Stavrakaki (AUA), Florian Schlenz (Geocledian), Simone Parisi CONTRIBUTORS