Evaluation of Grape and Wine Quality Parameters of Agiorgitiko
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Smell Incredible in 2021 Hello Parfume Lovers!
THE ULTIMATE GUIDE SMELL INCREDIBLE IN 2021 HELLO PARFUME LOVERS! The beauty and true power of perfumes is that they are deeply personal. They can evoke strong memo- ries with a single note. They can draw us to others, creating special bonds. And they can make us feel exactly like we want to feel; sexy, beautiful, relaxed or bossy. In this way fragrance is also a form of ex- pression, a way of revealing your mood or personal- ity, giving others a piece of yourself simply through smell. In this little booklet, we share a few interesting, fun and practical facts and tips about all the awesome the ways fragrance can have an impact on your ev- eryday life. 2 CONTENTS FACTS YOU SHOULD KNOW ABOUT PERFUME 4 HOW TO CHOOSE »THE« FRAGRANCE? 8 FRAGRANCE STRUCTURE 11 FRAGRANCE FAMILIES 14 CHOOSE YOUR PERFECT »SECOND SKIN« FRAGRANCE 17 HOW DO YOU WANT TO FEEL? 23 THE POWER OF FRAGRANCE: HEALTH BENEFITS 25 FASCINATING LINK BETWEEN FRAGRANCES, EMOTIONS AND 28 BEHAVIOUR THE FORCE OF FRAGRANCE-ATTRACTION 30 PERFUME: THE STORY OF A MURDERER 33 THINGS NO ONE EVER TELLS YOU ABOUT PERFUME: 36 PERFUME: from hand-pressed to fully personalized 40 READY FOR THE FUTURE OF COSMETICS 43 FIRST SMART PERFUME IN THE WORLD 45 3 FACTS YOU SHOULD KNOW ABOUT PERFUME Fragrance and perfume are an important part of our everyday lives, yet we do not know a lot about their origin, background and frankly, simple day-to-day, usage-related facts. So, we’d like to take you on a brief perfume history tour and translate some of the commonly unknown phrases we often hear when shopping for our perfect perfume. -

Artemis Karamolegos Wines Descriptions for Wine Experts
ARTEMIS KARAMOLEGOS WINES DESCRIPTIONS FOR WINE EXPERTS Having its roots in the volcanic soil of Santorini and tradition that goes back to 1952, the winery of Artemis Karamolegos is one of the most dynamic and rapidly evolving wineries of Santorini. All its wines have been distinguished in several important International Wine Competitions. The winery has the most modern facilities for wine production, spaces for wine testing and a shop for wines and selected local products. Artemis Karamolegos had the innovative idea to combine the experience of a tour at the winery with lunch at the restaurant Aroma Avlis, the menu of which has the signature of the talented chef Christos Coskinas. In its spacious new yard offering view to the vineyard and the beaches of Monolithos and Avis, as well as in the dinning halls, you can taste the delicious Mediterranean and local dishes made with fresh, carefully selected local products, accompanied with wines from the winery. The history of the winery goes back to the 1952, where the grandfather, Artemis, was cultivating the vineyards in order to produce wine for his own family and later on, in order to sell it in the island and in the rest of Greece. Artemis Karamolegos, the grandson who succeeded his grandfather and his father at the winery of Exo Gonia, is an energetic young man full of passion for his Job. Since 2004 and until today, he managed to lead, miraculously, the family business many steps ahead very fast. In 2004, a turn to a modern and of a high quality production winery took place, with the production of a bottled, labeled and of a good quality wine named “SANTORINI”. -

Wine Storage and Service Handbook
Wine storage and service handbook By EuroCave There is nothing more civilized than wine. François Rabelais Contents Wine – a great French tradition 7 Making wine 8 A guide to wine storage and preservation 22 Cabinets with state-of-the-art storage 24 Choose your wine cabinet according to your requirements 26 Wines and temperatures 27 EuroCave, inventor of the wine cabinet 31 EuroCave, the ideal solution to your wine storage requirements 32 About EuroCave wine cabinets 35 About EuroCave storage systems 51 EuroCave, wine cabinets with tailor-made layouts 60 The EuroCave application 62 Glossary 65 Wine - a great French tradition! Wine is an integral part of France’s culture, history and status. Recognised throughout the world for its wine production, which represents 20% of production worldwide and boasting a wealth of great wines renowned world- wide, France has been able to follow a way of life “steeped in the philosophy and culture of wine”. And the French are rather proud of this expertise… Today, there has been a change in their wine drinking habits: French consumers now drink less frequently but the wine is of better quality. Hence the increasing importance placed on wine cabinets. Wine is a living pro- duct: it must be stored in ideal conditions to optimize its development process. What are ideal conditions? A natural cellar, below ground, with stone walls, a vaulted ceiling and beaten- earth floor. However, natural cellars are not accessible to everyone: Should you therefore give up on the idea of having a wine cellar? Fortunately no – since EuroCave created the first electric wine cabinet! 7 Vine shoot Stalk Peduncle It is the framework of the Making wine bunch of grapes which above all contains tannins IN COLLABORATION WITH JOHN EUVRARD MOF (Meilleur Ouvrier de France) SOMMELIER France’s top sommelier RAW MATERIALS The vine The grape How is wine made? This fruit, Mediterranean in origin, is now grown almost everywhere throughout Tendril Wine is not “manufactured” it is produced. -
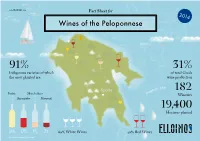
Wine Map of the Peleponnese 2014
www.ELLOINOS.com Fact Sheet for 2014 Wines of the Peloponnese Patras Athens 91% 31% Indigenous varieties of which of total Greek the most planted are: wine production n Sea Sparta gea 182 Ae Roditis Moschofilero Wineries Agiorgitiko Mavroudi 19,400 Hectares planted 34% 17% 9% 7% 60% White Wines 40% Red Wines Information design by ideologio Protected Designation of Origin Wine Colors Muscat of Rio Patras Grape: Muscat Blanc Mavrodaphne of Patras Grapes: Mavrodaphne, Korinthiaki Athens Nemea Grape Agiorgitiko Muscat of Patras Mantinia Grape: Muscat Blanc Grape Moschofilero Patras Epidaurus Grape: Roditis Sea ean eg Kalamata A In the EU, schemes of geographical indications known as Protected Designation of Origin (PDO) and Protected Monemvassia Geographical Indication (PGI), promote and protect names of —Malvasia quality agricultural and food products. Amongst many other products, the names of wines are also protected by these Grapes: Monemvassia (min 51%), laws. Assyrtiko, Asproudes, Kydonitsa PDO products are prepared, processed, and produced in a given geographical area, using recognized know-how and therefore acquire unique properties. White Wine Sweet White Wine Red Wine Sweet Red Wine Indigenous grapes International grapes Region Note There are additional grape varieties allowed, but PGI products are closely linked to the geographical current plantings are small. area in which they are traditionally and at least White indigenous: Asproudes Patras, Aidani, partially manufactured (prepared, processed OR Assyrtiko, Athiri, Glikerithra, Goustolidi, Laghorthi, produced), and have specific qualities attributable to Migdali, Petroulianos, Potamissi, Robola, Rokaniaris, Skiadopoulo, Sklava, Volitsa Aspro. that geographical area, therefore acquiring unique properties. Depending on their geographical breadth, Red indigenous: Limniona, Skylopnichtis, Thrapsa, Voidomatis, Volitsa. -

Installation Instructions and User Guide
Installation instructions and user guide Wine Cellar JC-110GD JC-160GD Contents Safety and warnings 2 Your Haier Wine Cellar 3 Installation instructions 4 Power supply 4 Positioning your Wine Cellar 4 5 5 Operating instructions 6 Storing red and white wine in the Wine Cellar 7 Short-term storage/serving 7 Long-term storage of wine 7 Interior fan 8 Ventilation control 8 Condensation control 8 Shelving 9 Deodoriser 9 Interior light 9 Cleaning and maintenance 10 Replacing the light bulb 11 Normal operating sounds you may hear 11 Transporting your Wine Cellar 11 Problem solving checklist 12 Important! SAVE THESE INSTRUCTIONS The model shown in this user guide may not be available in all markets and is subject to change at any time. For current details about model and specication availability in your country, please contact your local Haier dealer. 1 Safety and warnings Safety and warnings To reduce the risk of re, electric shock, or injury to persons, please read the IMPORTANT SAFETY INSTRUCTIONS before operating this appliance. Use this appliance only for its intended purpose as described in this User Guide. Important! When using this appliance, always exercise basic safety precautions including the following: This appliance is not intended for use by persons (including children) with reduced physical, sensory or mental capabilities, or lack of experience and knowledge, unless they have been given supervision or instruction concerning use of the appliance by a person responsible for their safety. Children should be supervised to ensure that they do not play with the appliance. Risk of child entrapment. -

J. Emerson, Inc. FINE WINES & CHEESE 5716 Grove Avenue•Richmond, VA
J. Emerson , Inc . FINE WINES & CHEESE 5716 Grove Avenue•Richmond, VA. 23226•(804) 285-8011 website – www.jemersonfinewine.com / Twitter: http://bit.ly/atAEL4 / Facebook: http://bit.ly/br4kEz STORE HOURS: Mon. 10:00 am to 8:00pm, Tues through Sat.: 9:00am to 8:00pm JANUARY 2011 NEWSLETTER NEW ARRIVALS •A CAL CAB SHORT SIPS • Practices & Policies th JOIN US ON THURSDAY JANUARY20 FOR A TASTING OF STAFF SELECTIONS 5:30 - 7:30pm at our store on Grove Ave. We’ll taste five of our favorite wines with a list available in the store and online. Discounts will be offered on all wines tasted. MONTHLY SPECIALS Jan. ‘10 USUAL CASE (cs.btl. cost) *2009 Castello di Uviglie "Bricco del Conte" Barbera del Monferrato (It.) $ 8.95 $10.95 $100 ($8.33) *2008 Vinska Klet "Giocato" Pinot Grigio (Brda, Slovenia) $ 9.95 $11.95 $110 ($9.17) *2008 Domaine Baron Touraine Cot (Loire Valley, France) $ 9.95 $11.95 $110 ($9.17) *2009 Domaine Baron Touraine Sauvignon Blanc (Loire Valley, Fr.) $ 9.95 $11.95 $110 ($9.17) 2007 La Cappucina "San Brizio" Soave (Veneto, Italy) $11.95 $14.95 $135 ($11.25) 2009 Castello di Uviglie Freisa (Piemonte, Italy) $12.95 $14.95 $135 ($11.25) 2009 Laurent Miquel "Nord-Sud" VdP Viognier (Languedoc, Fr.) $12.95 $14.95 $140 ($11.67) 2008 Franck Peillot Mondeuse de Bugey (Savoie, France) $14.95 $18.95 $160 ($13.33) A NOTE ABOUT OUR CASE OF THE MONTH: Our case of the month is an excellent way to get real value for your wine dollar and drink wines from around the world, carefully selected by J. -
Table of Contents
TABLE OF CONTENTS Sparkling & Champagne ............................. 3 White Wine .................................................. 4 Greece ........................................................................................4 Mediterranean ......................................................................5 Germany ...................................................................................5 Italy ................................................................................................5 Spain ........................................................................................... 6 France ........................................................................................ 6 From the New World .......................................................7 Rosé Wine ................................................ 8 Skin-Contact Wine ................................... 9 Red Wine .................................................10 Greece .............................................................................10 Mediterranean ...........................................................12 Italy ..................................................................................... 12 Spain .................................................................................. 13 France............................................................................... 13 From the New World ............................................ 14 Thrace Macedonia Epirius Thessaly Ionian Islands Aegean Peloponnese Islands Crete 2 SPARKLING -

WINES SELECTION Italian Law Quality Guarantee Marks
DRINK LIST OUR BEST WINES SELECTION Italian Law Quality Guarantee Marks D.O.C. Controlled Origin D.O.C.G. Controlled and Guaranteed Origin GREEK WINES WHITE WINES ESTATE P.D.O. OAK BARELS MORAITIS Winery: Moraitis Features: Dry white wine, 80% of the wine is fermented in a stainless steel vat with 6 months maturation on its lees. The rest Area of Origin: Paros , Greece 20% is fermented and matured for 6 months in new French oak barrels. Bright yellow gold color. Intense aromas of exotic fruits Grape Variety: 100% Monemvassia/ and citrus. The oak is well integrated and the crispness and elegan Vineyard Kamares ce of the wine easily dominates. Alcohol: 13 %vol. Price per Bottle 38 T S E KTIMA ALFA B E C C H OI Winery: Alpha Estate Area of Origin: Amyntaion, Greece Features: Bright white wine with intense flavor and rich aroma of citrus. Grape Variety: Sauvignon Blanc 100% Price per Bottle 29,5 Alcohol: 13,5 %vol. ESTATE MALAGOUSIA BIO MORAITIS Winery: Moraitis Features: Dry white wine, pale white yellow colour with green Area of Origin: Paros , Greece reflections. Rich in citrus and exotic fruit aromas. On the palate it is balanced, fruity, with notes of lemon peel and grapefruit. Grape Variety: Malagousia Price per Bottle 28,5 Alcohol: 12,5 %vol. CHARDONNAY GEROVASSILIU Winery: Gerovassiliu Area of Origin: Epanomi-Salonicco, Features: White wine aged in oak barrels, with a strong flavor and Greece characteristic with notes of citrus, dried fruit and vanilla. Grape Variety: Chardonnay Price per Bottle 33,5 Alcohol: 12,5 %vol. -

Grape and Wine Production in the Four Corners Region
Grape and Wine Production in the Four Corners Region Item Type text; Book Authors Mielke, Eugene A.; Dutt, Gordon R.; Hughes, Sam K.; Wolfe, Wade H.; Loeffler, Gregory J.; Gomez, Ricardo; Bryant, M. Douglas; Watson, John; Schick, Seth H. Publisher College of Agriculture, University of Arizona (Tucson, AZ) Rights Copyright © Arizona Board of Regents. The University of Arizona. Download date 03/10/2021 23:02:35 Link to Item http://hdl.handle.net/10150/602124 Technical Bulletin 239 University of Arizona Agricultural Experiment Station CORN% Eot S:;:, 9FC/ONAL COOS Grape and Wine Production in the Four Corners Region This is a report of research performed with financial assistance from the Four Corners Regional Commission Grape and Wine Production in the Four Corners Region UNIVERSITY OF ARIZONA TECHNICAL BULLETIN 239 REGIONAL PUBLICATION Eugene A. Mielke Gordon R. Dutt Sam K. Hughes Wade H. Wolfe University of Arizona Agricultural Experiment Station Gregory J. Loeffler Colorado State University Agricultural Experiment Station Ricardo Gomez M. Douglas Bryant John Watson New Mexico State University Seth,H, Schick Schick International, Inc. Salt Lake City, Utah CONTENTS Chapter Page INTRODUCTION 2 1 CLIMATE 3 Climatic Regions 4 Climatic Characterization of the Region 6 2 SOILS 24 Factors Affecting Soil Formation 25 Delineation of Grape- Growing Areas 28 Site Selection 31 3 VINEYARD ESTABLISHMENT 34 Land Preparation 35 Laying Out the Vineyard 35 Planting Stock 37 Propagation 38 4 TRAINING NEW VINEYARDS 41 Training 42 Pruning 46 Pruning Systems -

Download Full
chemistrychemistry September–November 2020 in Australia Fertile future for sustainable fuel chemaust.raci.org.au • The pressing concern of water security • Louis Pasteur, father of microbiology and virology • The problem of patenting polymorphs www.rowe.com.au Online 24 hours 7 days a week, by phone or face to face, we give you the choice. INSTRUMENTS - CONSUMABLES - CHEMICALS - SERVICE & REPAIRS A 100% Australian owned company, supplying scientific laboratories since 1987. South Australia & NT Queensland Victoria & Tasmania New South Wales Western Australia Ph: (08) 8186 0523 Ph: (07) 3376 9411 Ph: (03) 9701 7077 Ph: (02) 9603 1205 Ph: (08) 9302 1911 ISO 9001:2015 LIC 10372 [email protected] [email protected] [email protected] [email protected] [email protected] SAI Global REF535 X:\MARKETING\ADVERTISING\CHEMISTRY IN AUSTRALIA September–November 2020 36 cover story Towards the hydrogen–ammonia economy The race towards sustainable synthesis of ammonia is underway. 16 Used mainly in fertilisers, this simple molecule is predicted to become a big player in renewable energy exports and energy security, as a liquid fuel replacement for fossil fuels. iStockphoto/Petmal 20 High and dry: priorities for our water insecurity 4 Editorial For the driest inhabited continent on the planet, water security is a pressing 5 Your say concern. news & research 24 Louis Pasteur: his chemistry and microbiology 7 On the market After making landmark discoveries in optical isomerism, tireless chemist Louis 8 News Pasteur progressed to work that would mark him as the father of microbiology 12 Research and virology. members 29 New Fellow 29 Obituaries 38 views & reviews 32 Books 34 Technology & innovation 36 Environment 38 Science for fun 40 Grapevine 41 Letter from Melbourne 42 Cryptic chemistry 42 Events chemaust.raci.org.au editorial A visual treatment This year is the World Health Organization’s International Year of the Nurse and Midwife, which includes the 200th anniversary of Florence Nightingale’s birth. -

University of Oklahoma Graduate College
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE GEOGRAPHIES OF KNOWLEDGE IN THE INTERNATIONAL FRAGRANCE INDUSTRY A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY By BODO KUBARTZ Norman, Oklahoma 2009 GEOGRAPHIES OF KNOWLEDGE IN THE INTERNATIONAL FRAGRANCE INDUSTRY A DISSERTATION APPROVED FOR THE DEPARTMENT OF GEOGRAPHY BY ___________________________ Dr. Fred Shelley, Co-Chair ___________________________ Dr. Bret Wallach, Co-Chair ___________________________ Dr. Robert Cox ___________________________ Dr. Karl Offen ___________________________ Dr. Darren Purcell ___________________________ Dr. Laurel Smith ___________________________ Dr. Andrew Wood © Copyright by BODO KUBARTZ 2009 All Rights Reserved. ACKNOWLEDGEMENTS The ‘discovery of the world’ has been a stereotypical focus of human geography. However, the research helped me to discover a new world for myself. The fragrance industry delineates a rich landscape of sensorial stimulations. I appreciated not only the experience of smelling perfumes in different environments and the ‘look behind the scenes’ but the diversity of approaches to perfumery in this artistic industry. Therefore, my first ‘thank you’ goes to the industry experts that spend their precious time with me and opened their doors for me in France, Germany, and the United States. Thank you very much for introducing me to a world that you experience, develop, and change every day. Second, a dissertation is a long journey. This one has seen different companions until it came into being. It developed quite a bit over time. My friends in the United States and in Germany contributed through their critique, mental support, active interest, questions, guidance, and feedback. Thus, the second ‘thank you’ goes to all companions. -

Mansfield Dissertation FINAL
Characterization of Key Volatile Compounds in Red Table Wines Produced from Frontenac Grapes (Vitis spp.) A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Anna Katharine Mansfield IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Gary A. Reineccius, Advisor September 2008 © Anna Katharine Mansfield 2008 ACKNOWLEDGMENTS As with any work, crediting everyone who provided help and support would produce a document as long as the dissertation itself. This is true of this work, in particular, as my part-time student status meant that many people had to make small sacrifices so that I could find the time to complete my studies. First, I have to thank Dr. Gary Reineccius for encouraging me to attempt this feat and supporting me throughout. His humor, insight and occasional silliness were essential to any success that I’ve had in my flavor analysis endeavors. In addition to guiding me towards a solid scientific footing, Gary has helped me establish sound instinct for the place career takes in the larger scheme of things, and how to maintain that balance. In the Dept. of Food Science, I also received invaluable aid and advice from Jean- Paul Shirle-Keller, a man of infinite patience, astounding knowledge and great generosity. Much of what I know about MS analysis, I learned from him. In the sensory realm, Dr. Zata Vickers helped me wade through the morass of statistical analysis, though I usually left her office with the feeling that the ultimate lesson sensory evaluation teaches us is that humans are inconsistent and statistically not reproducible...and perhaps that’s a valuable thing to remember, after all.