Phenolic Compounds As Markers of Wine Quality and Authenticity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1.2 Weingartenflächen Und Flächenanteile Der Rebsorten EN
1. Vineyard areas and areas under vine by grape variety Austrian Wine statistics report 1.2 Vineyard areas and areas under vine by grape variety 2 The data in this section is based on the 2015 Survey of Area under Vine, as well as feedback from the wine-producing federal states of Niederösterreich (Lower Austria), Burgenland, Steiermark (Styria) and Wien (Vienna). The main source of data for the 2015 Survey of Area under Vine was the Wein-ONLINE system operated by the Federal Ministry of Agriculture, Forestry, Environment and Water Management (BMNT). Data from the remaining federal states was collected by means of a questionnaire (primary data collection). Information relating to the (approved) nurseries was provided by the Burgenland and Lower Austrian Chambers of Agriculture and the Styrian state government (Agricultural Research Centre). According to the 2015 Survey of Area under Vine, Austria’s vineyards occupied 45,574 hectares. The planted vineyard area was 45,439 ha, which corresponds to 94 ha less (or a 0.2% decrease) in comparison to the 2009 Survey of Area under Vine. The long-running trend that suggested a shift away from white wine and towards red was quashed by the 2015 Survey of Area under Vine. While the white wine vineyard area increased by 2.3% to 30,502 ha compared to 2009, the red wine area decreased by 4.9% to 14,937 ha. Figure 1 shows the evolution of Austrian viticulture after the Second World War. The largest area under vine was recorded in 1980 at 59,432 ha. From 1980 onwards, the white wine vineyard area has continuously decreased, while the red wine vineyard area has expanded. -

Artemis Karamolegos Wines Descriptions for Wine Experts
ARTEMIS KARAMOLEGOS WINES DESCRIPTIONS FOR WINE EXPERTS Having its roots in the volcanic soil of Santorini and tradition that goes back to 1952, the winery of Artemis Karamolegos is one of the most dynamic and rapidly evolving wineries of Santorini. All its wines have been distinguished in several important International Wine Competitions. The winery has the most modern facilities for wine production, spaces for wine testing and a shop for wines and selected local products. Artemis Karamolegos had the innovative idea to combine the experience of a tour at the winery with lunch at the restaurant Aroma Avlis, the menu of which has the signature of the talented chef Christos Coskinas. In its spacious new yard offering view to the vineyard and the beaches of Monolithos and Avis, as well as in the dinning halls, you can taste the delicious Mediterranean and local dishes made with fresh, carefully selected local products, accompanied with wines from the winery. The history of the winery goes back to the 1952, where the grandfather, Artemis, was cultivating the vineyards in order to produce wine for his own family and later on, in order to sell it in the island and in the rest of Greece. Artemis Karamolegos, the grandson who succeeded his grandfather and his father at the winery of Exo Gonia, is an energetic young man full of passion for his Job. Since 2004 and until today, he managed to lead, miraculously, the family business many steps ahead very fast. In 2004, a turn to a modern and of a high quality production winery took place, with the production of a bottled, labeled and of a good quality wine named “SANTORINI”. -

5.5.21 Wine! Copy.Pages
BUBBLES Adami, Prosecco di Valdobiaddene Superiore Bocca di Gica NV $58 Raventós i Blanc, Conca del Riu Anoia Brut Rose ‘de Nit’ 2017 $76 Ferghettina Franciacorta Brut NV, Lombardy, Italy $68 Champagne Drappier, Brut Blanc de Noir NV $105 Champagne Gaston-Chiquet “Tradition” Brut NV $60 (half bottle) Marc Hébrart, Champagne 1er Cru Brut Blanc de Blancs NV $120 WHITE WINE Medium and Crisp Josmeyer, Fromenteau Le Pinot Gris 2015 $81 Alsace, France Domaine Sigalas, Santorini Assyrtiko 2018 $72 Santorini, Greece Robert Weil Riesling Trocken Kiedrich Turmberg 2015 $100 Rheingau, Germany Taille Aux Loups Montlouis Sec “Clos de Monsy” 2016 $75 Loire Valley, France Chenin Blanc Bernhard Ott, "Fass 4" 2018 $80 Wagram, Austria Grüner Veltliner Bodegas Ordóñez S.L., “Nisia” Old Vines 2017 $61 Rueda, Spain Verdejo Domaine Vincent Dampt, Chablis 1er Cru Côte de Lechet 2018 $76 Burgundy, France Chardonnay Ciro Picariello, Fiano di Avellino 2018 $63 Campania, Italy Frog's Leap, Napa Valley Chardonnay (2017) $74 Napa Valley, California Stony Hill Vineyard, Chardonnay 2009 $118 Napa Valley, California WHITE WINE Rich and Full Emidio Pepe, Pecorino D’Abruzzo 2001 $250 Abruzzo, Italy Olivier Leflaive Chassagne-Montrachet Abbaye De Morgeot 2014 $195 Chardonnay RED WINE Light and Elegant Sottimano, Barbaresco ‘Fausoni’ 2015 $120 Piedmont, Italy Nebbiolo Castello di Verduno, Verduno Pelaverga Basadone 2018 $76 Piedmont, Italy Michel Magnien, Morey-Saint-Denis 1er Cru 'Les Chaffots' 2016 $183 Burgundy, France Pinot Noir Brewer-Clifton, Sta. Rita Hills Pinot -

SPARKLING WINES 5Oz 8Oz Bt Vendaval, Cuvée Reserve, Blanc De Blancs, Chardonnay, Curico Valley, Chile | NV 13 | 17 | 50
SPARKLING WINES 5oz 8oz Bt Vendaval, Cuvée Reserve, Blanc de Blancs, Chardonnay, Curico Valley, Chile | NV 13 | 17 | 50 Pierre Sparr, Crémant d’Alsace, Brut Rosé Reserve, Alsace, France | NV 17 | 23 | 66 Wine Spectator Canard Duchên, Brut, Montagne de Reims, Champagne, France | NV 22 | 35 | 86 Award Wine List Ca’ Stele, Extra Dry, Glera, Prosecco, Veneto, Italy | NV 13 | 18 | 50 Available Online Pitars, Brut, Rosé, Glera, Pinot Nero, Prosecco, Trentino, Italy | 2019 15 | 20 | 58 ROSÉ WINE Château Gassier, Esprit, Rosé, Grenache, Cinsault, Syrah, Rolle, Provence, France | 2019 14 | 19 | 54 Olé & Obrigado, Mencia, Bierzo, Spain | 2019 12 | 17 | 46 ORANGE WINE Scarbolo Ramato, Pinot Grigio, Alto Adige, Italy | 2018 17 | 24 | 66 Movia, Ribolla, Goriška Brda, Slovenia |2018 20 | 28 | 78 FUNK WINE Domaine Richaud, Grenache, Mourvedre, Syrah, Cotes du Rhone, France | 2018 17 | 25 | 66 WHITE WINES Paul Pernot et ses Fils, Aligoté, Côte-d’Or, France | 2018 18 | 25 | 70 Contesa, Pecorino, Abruzzo, Italy | 2018 12 | 17 | 46 Cascina Chicco, Anterisio, Arneis, Piedmont, Italy | 2018 14 | 19 | 54 San Salvatore, Paestum, Falanghina, Campania, Italy | 2018 16 | 21 | 62 Wildsong, Wildflower, Sauvignon Blanc, Hawke’s Bay, New Zealand | 2019 13 | 18 | 50 Vila Nova, Loureiro, Fernão Pires, Vinho Verde, Portugal | 2019 11 | 15 | 42 Naia, Verdejo, Rueda, Spain | 2019 13 | 18 | 50 Sandhi, Chardonnay, Central Coast, California | 2019 17 |23 | 66 RED WINES Tapiz, Alta Collection, Malbec, Valle de Uco, Mendoza, Argentina | 2019 14 | 19 | 54 Hugl Weine, Zweigelt, Niederösterreich, Burgenland, Austria | 2016 13 | 18 | 50 Domaine Vallot, Le Coriançon, Grenache, Syrah, Mourvèdre, Cotes-du-Rhone, France | 2019 13 | 18 | 50 Mauro Molino, Barolo, La Morra, Piedmont, Italy|2016 22 | 30 | 90 Rocim, Mariana, Touriga Nacional, Aragonez, Alicante Bouschet, Alentejo, Portugal | 2018 16 | 21 | 62 Carlos Serres, Gran Reserva, Tempranillo, Graciano, Mazuelo, Rioja, Spain | 2012 18 | 25 | 70 Samual Lindsay, The Gandy Dancer, Cabernet Sauvignon. -

The Cheeses Dolomites
THE CHEESES UNIONE EUROPEA REGIONE DEL VENETO OF THE BELLUNO DOLOMITES Project co-financed by the European Union, through the European Regional Development fund. Community Initiative INTERREG III A Italy-Austria. Project “The Belluno Cheese Route – Sights and Tastes to Delight the Visitor.” Code VEN 222065. HOW THEY ARE CREATED AND HOW THEY SHOULD BE ENJOYED HOW THEY ARE CREATED AND HOW THEY SHOULD BE ENJOYED HOW THEY ARE CREATED BELLUNO DOLOMITES OF THE CHEESES THE FREE COPY THE CHEESES OF THE BELLUNO DOLOMITES HOW THEY ARE CREATED AND HOW THEY SHOULD BE ENJOYED his booklet has been published as part of the regionally-managed project “THE BELLUNO CHEESE ROUTE: SIGHTS AND TASTES TO TDELIGHT THE VISITOR”, carried out by the Province of Belluno and the Chamber of Commerce of Belluno (with the collaboration of the Veneto Region Milk Producers’ Association) and financed under the EU project Interreg IIIA Italy-Austria. As is the case for all cross-border projects, the activities have been agreed upon and developed in partnership with the Austrian associations “Tourismusverband Lienzer Dolomiten” (Lienz- Osttirol region), “Tourismusverband Hochpustertal” (Sillian) and “Verein zur Förderung des Stadtmarktes Lienz”, and with the Bolzano partner “Centro Culturale Grand Hotel Dobbiaco”. The project is an excellent opportunity to promote typical mountain produce, in particular cheeses, in order to create a close link with the promotion of the local area, culture and tourism. There is a clear connection between, one the one hand, the tourist, hotel and catering trades and on the other, the safeguarding and promotion of typical quality produce which, in particular in mountain areas, is one of the main channels of communication with the visitor, insofar as it is representative of the identity of the people who live and work in the mountains. -
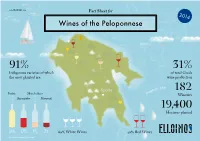
Wine Map of the Peleponnese 2014
www.ELLOINOS.com Fact Sheet for 2014 Wines of the Peloponnese Patras Athens 91% 31% Indigenous varieties of which of total Greek the most planted are: wine production n Sea Sparta gea 182 Ae Roditis Moschofilero Wineries Agiorgitiko Mavroudi 19,400 Hectares planted 34% 17% 9% 7% 60% White Wines 40% Red Wines Information design by ideologio Protected Designation of Origin Wine Colors Muscat of Rio Patras Grape: Muscat Blanc Mavrodaphne of Patras Grapes: Mavrodaphne, Korinthiaki Athens Nemea Grape Agiorgitiko Muscat of Patras Mantinia Grape: Muscat Blanc Grape Moschofilero Patras Epidaurus Grape: Roditis Sea ean eg Kalamata A In the EU, schemes of geographical indications known as Protected Designation of Origin (PDO) and Protected Monemvassia Geographical Indication (PGI), promote and protect names of —Malvasia quality agricultural and food products. Amongst many other products, the names of wines are also protected by these Grapes: Monemvassia (min 51%), laws. Assyrtiko, Asproudes, Kydonitsa PDO products are prepared, processed, and produced in a given geographical area, using recognized know-how and therefore acquire unique properties. White Wine Sweet White Wine Red Wine Sweet Red Wine Indigenous grapes International grapes Region Note There are additional grape varieties allowed, but PGI products are closely linked to the geographical current plantings are small. area in which they are traditionally and at least White indigenous: Asproudes Patras, Aidani, partially manufactured (prepared, processed OR Assyrtiko, Athiri, Glikerithra, Goustolidi, Laghorthi, produced), and have specific qualities attributable to Migdali, Petroulianos, Potamissi, Robola, Rokaniaris, Skiadopoulo, Sklava, Volitsa Aspro. that geographical area, therefore acquiring unique properties. Depending on their geographical breadth, Red indigenous: Limniona, Skylopnichtis, Thrapsa, Voidomatis, Volitsa. -

Weissweine Aus Der Bodenseeregion
WEISSWEINE AUS DER BODENSEEREGION Weine vom Weingut Möth in Bregenz Die besten und größten Lagen befinden sich in unmittelbarer Nähe zum Bodensee in der Lage Neu Amerika. Hier profitiert man von den klimatischen Gegebenheiten des großen Sees (Wärmespeicher, Reflexionswärme, Fön). Die Reben stehen auf sandigem Kiesboden. Mit etwa 3,5 ha ist dieser Weinbaubetrieb der größte und auch einzige, der als Haupterwerb in Vorarlberg geführt wird. Chardonnay 2018 34.00 Im Duft leicht rauchig, würzig, am Gaumen cremig, mineralisch, mit zarter Säuretextur. Seebrünzler 2018 32.00 Duftig, fruchtige Nase nach Pfirsich und Muskat. Am Gaumen Fruchtsüsse mit weicher Säure unterlegt. Weingut Aufricht in Stetten Das Weingut Aufricht ist ein privates Weingut der Brüder Robert und Manfred Aufricht im Weinanbaugebiet Baden mit Sitz in Stetten am Bodensee. Es bewirtschaftet eine Ertragsrebfläche von 35 Hektar und produziert von 250.000 bis 300.000 Flaschen Wein. Auxerrois 2018 35.00 Faszinierende gelbe Steinfrucht, cremig unterlegt von einem dezenten, weichen Pinot-Ton. Angenehm wenig Säure. Winzerverein Hagnau Der Winzerverein Hagnau ist die älteste Winzergenossenschaft im Weinanbaugebiet Baden mit Sitz in Hagnau am Bodensee. Er bewirtschaftet eine Ertragsrebfläche von 166 Hektar. Müller Thurgau „Felchen“ 2017 | 2018 27.00 Hagnauer Burgstall WEISSWEINE AUS ÖSTERREICH In allen österreichischen Weinbauregionen gehen kleine, aber auch größere Winzer den Weg der kompromisslosen Qualität und finden damit großen Anklang. Einige der Besten davon haben wir für Sie ausgesucht. -

7 Elements Introduction to Austrian Wine
7 ELEMENTS INTRODUCTION TO AUSTRIAN WINE © AWMB SEVEN ELEMENTS OF UNIQUENESS Key Facts © AWMB/Philipp Forster AUSTRIAN VINEYARD AREA IN 2015 Total: 46,515 ha / 115.000 acres BASIC FACTS • Production: 250 million litres • Consumption: 250 million litres • Import: 50 – 70 million litres • Export: 50 – 70 million litres AUSTRIA‘S BOOMING WINE EXPORTS Value: € 170 million Volume: 53 million litres Ø Price: 3.24 Euro/liter Source: Statistics Austria, preliminary export figures I-XII 2018 (as of March 2019). The data capture method used by Statistics Austria also includes re-exports of non-Austrian wine. 1995 no data available. AUSTRIAN WINE EXPORTS: BOTTLE VS. BULK 65.000 60.000 55.000 50.000 45.000 40.000 35.000 30.000 25.000 inL 1.000 20.000 15.000 10.000 5.000 0 2000 2001 2002 2003 2011 2012 2013 2014 2015 2016 2017 2004 2005 2006 2007 2008 2009 2010 MengeBottles Flasche MengeBulk Fass AUSTRIA‘S TOP 10 EXPORT MARKETS (REVENUE) Source: Statistik Austria, preliminary export numbers for 2018; March 2019 1. THE CLIMATE 2. THE LAND 3. THE GRAPES 4. THE CULTURE 5. NATURE 6. VALUE FOR MONEY 7. THE TASTE © AWMB/Philipp Forster 1. THE CLIMATE 2. THE LAND 3. THE GRAPES 4. THE CULTURE 5. NATURE 6. VALUE FOR MONEY 7. THE TASTE © AWMB/Anna Stöcher ([email protected]) THE TENSION OF OPPOSITES Skiing in the West Wine in the East © AWMB © AWMB THE TENSION OF OPPOSITES 3 2 1 1. Continental-pannonian 2. Temperate atlantic 4 3. Cool air from the north 4. -

Wines of Alentejo Varieties by Season Sustainability Program (WASP) 18 23 24
Alentejo History Alentejo The 8 sub-regions of DOC the 'Alentejo' PDO 2 6 8 'Alentejano' Grape Red Grape PGI Varieties Varieties 10 13 14 The Alentejo White Grape Viticulture Season Wines of Alentejo Varieties by Season Sustainability Program (WASP) 18 23 24 Wine Tourism Alentejo Wine Grapes used in Gastronomy Wines of Alentejo blends 26 28 30 Facts and Guarantee Figures of Origin 33 36 WINES OF ALENTEJO UNIQUE BY NATURE CVRA - COMISSÃO VITIVINÍCOLA REGIONAL ALENTEJANA Copy: Rui Falcão Photographic credits: Nuno Luis, Tiago Caravana, Pedro Moreira and Fabrice Demoulin Graphic design: Duas Folhas With thanks to Essência do Vinho The AlentejoWINE REGION There is something profoundly invigorating and liberating about the Alentejo landscape: its endlessly open countryside, gently undulating plains, wide blue skies and distant horizons. The landscape mingles with the vines and cereal crops – an ever-changing canvas of colour: intensely green towards the end of winter, the colour of straw at the end of spring, and deep ochre during the final months of summer. 1 All over the Alentejo there are archaeological markers suggesting that wine has Historybeen an important part of life up to the present day. Whilst it is not known exactly when wine and viticulture was introduced to the Alentejo, there is plenty of evidence that they were already part of the day-to-day life in the Alentejo by the time the Romans arrived in the south of Portugal. It is thought that the Tartessians, an ancient civilisation based in the south of the Iberian Peninsula and heirs of the Andalusian Megalithic culture, were the first to domesticate vineyards and introduce winemaking principles in the Alentejo. -

Brut Champagne
BY THE GLASS Sparkling DOMAINE CHANDON BRUT, CALIFORNIA $14 LUCA PARETTI ROSE SPUMANTE, TREVISO, ITALY $16 MOET BRUT IMPERIAL, ÉPERNEY, FRANCE $24 PERRIER JOUET GRAND BRUT, CHAMPAGNE, FRANCE $26 VEUVE CLICQUOT YELLOW LABEL, REIMS, FRANCE $28 Sake SUIGEI TOKUBETSU “DRUNKEN WHALE”, JUNMAI $13 FUNAGUCHI KIKUSUI ICHIBAN, HONJOZO (CAN) $14 KAMOIZUMI “SUMMER SNOW” NIGORI (UNFILTERED) $21 SOTO JUNMAI DAIGINJO $18 HEAVENSAKE JUNMAI DAIGINJO $18 Rose VIE VITE “WHITE LABEL”, CÔTES DE PROVENCE, FRANCE $15 Whites 13 CELSIUS SAUVIGNON BLANC, NEW ZEALAND $15 50 DEGREE RIESLING, RHEINGAU, GERMANY $15 WENTE VINEYARDS ”RIVA RANCH” CHARDONNAY, ARROYO SECO, MONTEREY $15 RAMON BILBAO ALBARIÑO, RIAS BAIXAS, SPAIN $15 ANTINORI “GUADO AL TASSO” VERMENTINO, BOLGHERI, ITALY $17 BARTON GUESTIERE “PASSEPORT”, SANCERRE, FRANCE $22 DAOU ”RESERVE” CHARDONNAY, PASO ROBLES, CALIFORNIA $24 Reds TRAPICHE “OAK CASK” MALBEC, MENDOZA, ARGENTINA $14 SANFORD “FLOR DE CAMPO” PINOT NOIR, SANTA BARBARA, CALIFORNIA $16 NUMANTHIA TINTO DE TORO, TORO, SPAIN $17 SERIAL CABERNET SAUVIGNON, PASO ROBLES $17 “LR” BY PONZI VINEYARDS PINOT NOIR, WILLAMETTE VALLEY, OREGON $22 ANTINORI “GUADO AL TASSO” IL BRUCIATO, BOLGHERI, ITALY $24 JORDAN WINERY CABERNET SAUVIGNON, ALEXANDER VALLEY, CALIFORNIA $32 “OVERTURE” BV OPUS ONE NAPA VALLEY, CALIFORNIA $60 Beers HOUSE BEER LAGER $8 STELLA ARTOIS PILSNER $8 GOOSE ISLAND SEASONAL $8 HEINEKEN/LIGHT LAGER $8 GOLDEN ROAD HEFEWEIZEN $8 LAGUNITAS LITTLE SUMPIN’ SUMPIN’ ALE $8 bubbles Brut ARMAND DE BRIGNAC “ACE OF SPADES”, CHAMPAGNE, NV $650 DELAMOTTE -
Table of Contents
TABLE OF CONTENTS Sparkling & Champagne ............................. 3 White Wine .................................................. 4 Greece ........................................................................................4 Mediterranean ......................................................................5 Germany ...................................................................................5 Italy ................................................................................................5 Spain ........................................................................................... 6 France ........................................................................................ 6 From the New World .......................................................7 Rosé Wine ................................................ 8 Skin-Contact Wine ................................... 9 Red Wine .................................................10 Greece .............................................................................10 Mediterranean ...........................................................12 Italy ..................................................................................... 12 Spain .................................................................................. 13 France............................................................................... 13 From the New World ............................................ 14 Thrace Macedonia Epirius Thessaly Ionian Islands Aegean Peloponnese Islands Crete 2 SPARKLING -

Untersuchung Der Transkriptionellen Regulation Von Kandidatengenen Der Pathogenabwehr Gegen Plasmopara Viticola in Der Weinrebe
Tina Moser Institut für Rebenzüchtung Untersuchung der transkriptionellen Regulation von Kandidatengenen der Pathogenabwehr gegen Plasmopara viticola in der Weinrebe Dissertationen aus dem Julius Kühn-Institut Julius Kühn-Institut Bundesforschungsinstitut für Kulturpfl anzen Kontakt/Contact: Tina Moser Arndtstraße 6 67434 Neustadt Die Schriftenreihe ,,Dissertationen aus dem Julius Kühn-lnstitut" veröffentlicht Doktorarbeiten, die in enger Zusammenarbeit mit Universitäten an lnstituten des Julius Kühn-lnstituts entstanden sind The publication series „Dissertationen aus dem Julius Kühn-lnstitut" publishes doctoral dissertations originating from research doctorates completed at the Julius Kühn-Institut (JKI) either in close collaboration with universities or as an outstanding independent work in the JKI research fields. Der Vertrieb dieser Monographien erfolgt über den Buchhandel (Nachweis im Verzeichnis lieferbarer Bücher - VLB) und OPEN ACCESS im lnternetangebot www.jki.bund.de Bereich Veröffentlichungen. The monographs are distributed through the book trade (listed in German Books in Print - VLB) and OPEN ACCESS through the JKI website www.jki.bund.de (see Publications) Wir unterstützen den offenen Zugang zu wissenschaftlichem Wissen. Die Dissertationen aus dem Julius Kühn-lnstitut erscheinen daher OPEN ACCESS. Alle Ausgaben stehen kostenfrei im lnternet zur Verfügung: http://www.jki.bund.de Bereich Veröffentlichungen We advocate open access to scientific knowledge. Dissertations from the Julius Kühn-lnstitut are therefore published open