High‐Throughput Sequencing Outperforms Traditional Morphological Methods in Blue Catfish Diet Analysis and Reveals Novel Insig
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
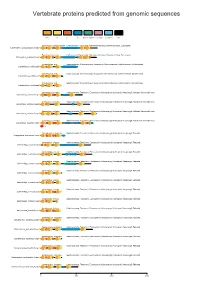
Vertebrate Proteins Predicted from Genomic Sequences
Vertebrate proteins predicted from genomic sequences VWD C8 TIL PTS Mucin2_WxxW F5_F8_type_C FCGBP_N VWC Lethenteron_camtschaticum Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Lethenteron Lethenteron_camtschaticum.0.pep1 Petromyzon_marinus Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Petromyzon Petromyzon_marinus.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep2 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep1 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep2 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.1.pep1 TILa Cynoglossus_semilaevis Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Teleostei; Cynoglossus_semilaevis.1.pep1 -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

The Genetic Factors of Bilaterian Evolution Peter Heger1*, Wen Zheng1†, Anna Rottmann1, Kristen a Panfilio2,3, Thomas Wiehe1
RESEARCH ARTICLE The genetic factors of bilaterian evolution Peter Heger1*, Wen Zheng1†, Anna Rottmann1, Kristen A Panfilio2,3, Thomas Wiehe1 1Institute for Genetics, Cologne Biocenter, University of Cologne, Cologne, Germany; 2Institute for Zoology: Developmental Biology, Cologne Biocenter, University of Cologne, Cologne, Germany; 3School of Life Sciences, University of Warwick, Gibbet Hill Campus, Coventry, United Kingdom Abstract The Cambrian explosion was a unique animal radiation ~540 million years ago that produced the full range of body plans across bilaterians. The genetic mechanisms underlying these events are unknown, leaving a fundamental question in evolutionary biology unanswered. Using large-scale comparative genomics and advanced orthology evaluation techniques, we identified 157 bilaterian-specific genes. They include the entire Nodal pathway, a key regulator of mesoderm development and left-right axis specification; components for nervous system development, including a suite of G-protein-coupled receptors that control physiology and behaviour, the Robo- Slit midline repulsion system, and the neurotrophin signalling system; a high number of zinc finger transcription factors; and novel factors that previously escaped attention. Contradicting the current view, our study reveals that genes with bilaterian origin are robustly associated with key features in extant bilaterians, suggesting a causal relationship. *For correspondence: [email protected] Introduction The taxon Bilateria consists of multicellular animals -

A Phylogenomic Perspective on the Radiation of Ray-Finned Fishes Based Upon Targeted Sequencing of Ultraconserved Elements
1 A phylogenomic perspective on the radiation of 2 ray-finned fishes based upon targeted sequencing of 3 ultraconserved elements 1;2;∗ 1;2 1 1 4 Michael E. Alfaro , Brant C. Faircloth , Laurie Sorenson , Francesco Santini 1 5 Department of Ecology and Evolutionary Biology, University of California, Los Angeles, CA, USA 2 6 These authors contributed equally to this work 7 ∗ E-mail: [email protected] arXiv:1210.0120v1 [q-bio.PE] 29 Sep 2012 1 8 Summary 9 Ray-finned fishes constitute the dominant radiation of vertebrates with over 30,000 species. 10 Although molecular phylogenetics has begun to disentangle major evolutionary relationships 11 within this vast section of the Tree of Life, there is no widely available approach for effi- 12 ciently collecting phylogenomic data within fishes, leaving much of the enormous potential 13 of massively parallel sequencing technologies for resolving major radiations in ray-finned 14 fishes unrealized. Here, we provide a genomic perspective on longstanding questions regard- 15 ing the diversification of major groups of ray-finned fishes through targeted enrichment of 16 ultraconserved nuclear DNA elements (UCEs) and their flanking sequence. Our workflow 17 efficiently and economically generates data sets that are orders of magnitude larger than 18 those produced by traditional approaches and is well-suited to working with museum speci- 19 mens. Analysis of the UCE data set recovers a well-supported phylogeny at both shallow and 20 deep time-scales that supports a monophyletic relationship between Amia and Lepisosteus 21 (Holostei) and reveals elopomorphs and then osteoglossomorphs to be the earliest diverging 22 teleost lineages. -

Direct Identification of Fish Species by Surface Molecular Transferring
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2020 Supplementary materials for Direct Identification of Fish Species by Surface Molecular Transferring Mingke Shao, Hongyan Bi* College of Food Science and Engineering, Shanghai Ocean University, Hucheng Ring Road 999, Pudong New District, 201306 Shanghai, China * To whom correspondence should be addressed. E-mail address: [email protected] E-mail address for the other authors: [email protected] S1 S1. Photos and information on the analyzed fish samples Fig. S1. Photos of fishes analyzed in the present study: (A) Oreochromis mossambicus (B) Epinephelus rivulatus (C) Mugil cephalus; (D) Zeus faber (E) Trachinotus ovatus (F) Brama japonica (G) Larimichthys crocea (H) Larimichthys polyactis (I) Pampus argenteus. Scale bar in each photo represents 1 cm. Table S1. List of the scientific classification of fishes analyzed in the study. The classification of fishes refers to https://www.fishbase.de/. Binomial Abbreviatio English Chinese name n common Scientific classification name (Scientific name name) Actinopterygii (class) > Perciformes (order) > Japanese Brama Brama BJ Bramidae (family) > Wufang japonica japonica Brama (genus) > B. brama (species) Actinopterygii (class) > Silver Scombriformes(order) > Baichang pomfret; Pampus PA ( Fish White argenteus Stromateidae family) > pomfret Pampus (genus) > P. argenteus (species) Haifang Zeus faber Actinopterygii (class) > (commonly Linnaeu; Zeus faber ZF Zeiformes (order) > called: John Dory; Zeidae (family) > S2 Yueliang target perch Zeus (genus) > Fish) Z. faber (species) Actinopteri (class) > OM Cichliformes (order) > Mozambique Oreochromis Cichlidae (family) > Luofei Fish tilapia mossambicus Oreochromis (genus) > O.mossambicus (species) Actinopterygii (class) > MC Mugiliformes (order) Xiaozhai Flathead Mugil Mugilidae (family) > Fish grey mullet cephalus Mugil (genus) > M. -

From the Middle Triassic of Guizhou, China
第53卷 第1期 古 脊 椎 动 物 学 报 pp. 1-15 2015年1月 VERTEBRATA PALASIATICA figs. 1-6 Panxianichthys imparilis gen. et sp. nov., a new ionoscopiform (Halecomorphi) from the Middle Triassic of Guizhou, China XU Guang-Hui1,2 SHEN Chen-Chen1 (1 Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences Beijing 100044 [email protected]) (2 State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) Nanjing 210008) Abstract The Ionoscopiformes are a fossil lineage of halecomorphs known only from the Mesozoic marine deposits. Because of their close relationships with the Amiiformes, the Ionoscopiformes are phylogenetically important in investigating the early evolution and biogeography of the Halecomorphi. However, fossil evidence of early ionoscopiforms was scarce; until recently, Robustichthys from the Middle Triassic Luoping Biota, eastern Yunnan, China, represents the oldest and only known ionoscopiform in the Triassic. Here we report the discovery of a new ionoscopiform, Panxianichthys imparilis gen. et sp. nov., on the basis of two well preserved specimens from the Middle Triassic Panxian Biota, western Guizhou, China. The discovery documents the second ionoscopiform in the Middle Triassic; although Panxianichthys is slightly younger than Robustichthys, it is significantly older than other members of this group from the Late Jurassic of Europe, and Early Cretaceous of North and South America. Panxianichthys possesses an important synapomorphy of the Ionoscopiformes: a sensory canal in the maxilla, but retains some primitive characters unknown in other ionoscopiforms. Results of our phylogenetic analysis recover Panxianichthys as the most primitive ionoscopiform, and provide new insight on the early evolution of this clade. -

The Living Planet Index (Lpi) for Migratory Freshwater Fish Technical Report
THE LIVING PLANET INDEX (LPI) FOR MIGRATORY FRESHWATER FISH LIVING PLANET INDEX TECHNICAL1 REPORT LIVING PLANET INDEXTECHNICAL REPORT ACKNOWLEDGEMENTS We are very grateful to a number of individuals and organisations who have worked with the LPD and/or shared their data. A full list of all partners and collaborators can be found on the LPI website. 2 INDEX TABLE OF CONTENTS Stefanie Deinet1, Kate Scott-Gatty1, Hannah Rotton1, PREFERRED CITATION 2 1 1 Deinet, S., Scott-Gatty, K., Rotton, H., Twardek, W. M., William M. Twardek , Valentina Marconi , Louise McRae , 5 GLOSSARY Lee J. Baumgartner3, Kerry Brink4, Julie E. Claussen5, Marconi, V., McRae, L., Baumgartner, L. J., Brink, K., Steven J. Cooke2, William Darwall6, Britas Klemens Claussen, J. E., Cooke, S. J., Darwall, W., Eriksson, B. K., Garcia Eriksson7, Carlos Garcia de Leaniz8, Zeb Hogan9, Joshua de Leaniz, C., Hogan, Z., Royte, J., Silva, L. G. M., Thieme, 6 SUMMARY 10 11, 12 13 M. L., Tickner, D., Waldman, J., Wanningen, H., Weyl, O. L. Royte , Luiz G. M. Silva , Michele L. Thieme , David Tickner14, John Waldman15, 16, Herman Wanningen4, Olaf F., Berkhuysen, A. (2020) The Living Planet Index (LPI) for 8 INTRODUCTION L. F. Weyl17, 18 , and Arjan Berkhuysen4 migratory freshwater fish - Technical Report. World Fish Migration Foundation, The Netherlands. 1 Indicators & Assessments Unit, Institute of Zoology, Zoological Society 11 RESULTS AND DISCUSSION of London, United Kingdom Edited by Mark van Heukelum 11 Data set 2 Fish Ecology and Conservation Physiology Laboratory, Department of Design Shapeshifter.nl Biology and Institute of Environmental Science, Carleton University, Drawings Jeroen Helmer 12 Global trend Ottawa, ON, Canada 15 Tropical and temperate zones 3 Institute for Land, Water and Society, Charles Sturt University, Albury, Photography We gratefully acknowledge all of the 17 Regions New South Wales, Australia photographers who gave us permission 20 Migration categories 4 World Fish Migration Foundation, The Netherlands to use their photographic material. -

VIRION Manuscript
bioRxiv preprint doi: https://doi.org/10.1101/2021.08.06.455442; this version posted August 9, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. The Global Virome in One Network (VIRION): an atlas of vertebrate-virus associations Colin J. Carlson1,2,3,* , Rory J. Gibb4,5, Gregory F. Albery3, Liam Brierley6, Ryan P. Connor7, Tad A. Dallas8,9, Evan A. Eskew10, Anna C. Fagre11,12, Maxwell J. Farrell13, Hannah K. Frank14, Renata L. Muylaert15, Timothée Poisot16,17, Angela L. Rasmussen2,18, Sadie J. Ryan19,20,21, and Stephanie N. Seifert22 1. Department of Microbiology and Immunology, Georgetown University Medical Center, Georgetown University, Washington, D.C., U.S.A. 2. Center for Global Health Science and Security, Georgetown University Medical Center, Georgetown University, Washington, D.C., U.S.A. 3. Department of Biology, Georgetown University, Washington, D.C., U.S.A. 4. Centre for Mathematical Modelling of Infectious Diseases, London School of Hygiene and Tropical Medicine, London, UK. 5. Centre on Climate Change and Planetary Health, London School of Hygiene and Tropical Medicine, London, UK. 6. Department of Health Data Science, University of Liverpool, Liverpool, UK. 7. National Center for Biotechnology Information, U.S. National Library of Medicine, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20894, U.S.A. 8. Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70806, U.S.A. -

The Evolutionary Transition from Lungs to a Gas Bladder: Evidence from Immunohistochemistry, Rna-Seq, and Morphology
THE EVOLUTIONARY TRANSITION FROM LUNGS TO A GAS BLADDER: EVIDENCE FROM IMMUNOHISTOCHEMISTRY, RNA-SEQ, AND MORPHOLOGY A Dissertation Presented to the Faculty of the Graduate School of Cornell University In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Emily Funk December 2020 © 2020 Emily Funk THE EVOLUTIONARY TRANSITION FROM LUNGS TO A GAS BLADDER: EVIDENCE FROM IMMUNOHISTOCHEMISTRY, RNA-SEQ, AND MORPHOLOGY Emily Funk, Ph. D. Cornell University 2020 Key to understanding the evolutionary origin and modification of phenotypic traits is revealing the underlying developmental genetic mechanisms. An important morphological trait of ray-finned fishes is the gas bladder, an air-filled organ that, in most fishes, functions for buoyancy control, and is homologous to the lungs of lobe- finned fishes. While gas bladders and lungs are similar in many ways, the distinguishing morphological difference between these organs is the general direction of budding from the foregut during development. Lungs bud ventrally and the gas bladder buds dorsally from the foregut endoderm. To compare lung and gasbladder development, the relevant taxa include bichir and bowfin. Bichir are the only living ray-finned fish that develops ventrally budding lungs. Bowfin, an early-diverging lineage, sister to teleosts, develops a gas bladder and exhibits a number of ancestral characteristics. Additionally, we included zebrafish as a representative of teleost fishes. I investigated the genetic underpinnings of this ventral-to-dorsal shift in budding direction using immunohistochemistry and RNA sequencing to determine whether gene expression patterns show a dorsoventral inversion paralleling the morphological inversion in budding direction. I also characterize morphological budding direction in bowfin, a purported transitional form, using nano-CT scanning. -

Family-Group Names of Fossil Fishes
European Journal of Taxonomy 466: 1–167 ISSN 2118-9773 https://doi.org/10.5852/ejt.2018.466 www.europeanjournaloftaxonomy.eu 2018 · Van der Laan R. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:1F74D019-D13C-426F-835A-24A9A1126C55 Family-group names of fossil fishes Richard VAN DER LAAN Grasmeent 80, 1357JJ Almere, The Netherlands. Email: [email protected] urn:lsid:zoobank.org:author:55EA63EE-63FD-49E6-A216-A6D2BEB91B82 Abstract. The family-group names of animals (superfamily, family, subfamily, supertribe, tribe and subtribe) are regulated by the International Code of Zoological Nomenclature. Particularly, the family names are very important, because they are among the most widely used of all technical animal names. A uniform name and spelling are essential for the location of information. To facilitate this, a list of family- group names for fossil fishes has been compiled. I use the concept ‘Fishes’ in the usual sense, i.e., starting with the Agnatha up to the †Osteolepidiformes. All the family-group names proposed for fossil fishes found to date are listed, together with their author(s) and year of publication. The main goal of the list is to contribute to the usage of the correct family-group names for fossil fishes with a uniform spelling and to list the author(s) and date of those names. No valid family-group name description could be located for the following family-group names currently in usage: †Brindabellaspidae, †Diabolepididae, †Dorsetichthyidae, †Erichalcidae, †Holodipteridae, †Kentuckiidae, †Lepidaspididae, †Loganelliidae and †Pituriaspididae. Keywords. Nomenclature, ICZN, Vertebrata, Agnatha, Gnathostomata. -

Resolution of Ray-Finned Fish Phylogeny and Timing of Diversification
Resolution of ray-finned fish phylogeny and timing of diversification Thomas J. Neara,1, Ron I. Eytana, Alex Dornburga, Kristen L. Kuhna, Jon A. Mooreb, Matthew P. Davisc, Peter C. Wainwrightd, Matt Friedmane, and W. Leo Smithc aDepartment of Ecology and Evolutionary Biology and Peabody Museum of Natural History, Yale University, New Haven, CT 06520; bWilkes Honors College, Florida Atlantic University, Jupiter, FL 33458; cDepartment of Zoology, Fishes, The Field Museum, Chicago, IL 60605; dDepartment of Evolution and Ecology, University of California, Davis, CA 95616; and eDepartment of Earth Sciences, University of Oxford, Oxford OX1 3AN, United Kingdom Edited by David M. Hillis, University of Texas, Austin, TX, and approved July 19, 2012 (received for review April 22, 2012) Ray-finned fishes make up half of all living vertebrate species. (11, 12)] are the sister lineage of all other teleosts. Second, the Nearly all ray-finned fishes are teleosts, which include most relationships of lower euteleosts (e.g., salmons, smelts, pikes, commercially important fish species, several model organisms for slickheads, and galaxiids), or “protacanthopterygians,” has changed genomics and developmental biology, and the dominant compo- frequently as a result of phylogenetic analyses of different mor- nent of marine and freshwater vertebrate faunas. Despite the phological datasets (13–15). Third, with at least 16,950 species (2), economic and scientific importance of ray-finned fishes, the lack the staggering diversity of spiny-rayed fishes, and particularly per- of a single comprehensive phylogeny with corresponding diver- comorphs, has impeded phylogenetic resolution of this lineage, gence-time estimates has limited our understanding of the evolu- prompting Nelson (16) to label the Percomorpha as the “bush at tion and diversification of this radiation. -

Animal Diversity in the Mangrove Forest at Bichitrapur of Balasore District, Odisha, India- a Case Study
Rec. zool. Surv. India: Vol. 119(1)/ 9-17, 2019 ISSN (Online) : 2581-8686 DOI: 10.26515/rzsi/v119/i1/2019/122954 ISSN (Print) : 0375-1511 Animal diversity in the mangrove forest at Bichitrapur of Balasore district, Odisha, India- A case study Santanu Mitra, Sayantani Shaw and Subhrendu Sekhar Mishra* Zoological survey of India, F.P.S. Building, 27 Jawaharlal Nehru Road, Kolkata – 700016, West Bengal, India: [email protected] Abstract The present work forms the preliminary study of ecosystem of Bichitrapur in Balasore district, though it covers a small area. A total 56 species of estuarine animals are found in the locality. They are normally mangrove associate animals, found in and over mangrove plants as borers or non-borers. These animals are either harmful to mangrove plants or opportunists by depending on mangroves for shelter or feeding. The dominating group in this mangrove is found to be the Molluscs, comprising 8 species of borers of mangrove-wood and 19 species as opportunistic epi-fauna. Crustaceans are the second diverse group in this ecosystem, represented by 13 species of crabs, 6 species of boring Isopods and a single species of Amphipod. The other epifaunal components comprise a few species of Cnidaria, Polychaetea, Echinoderm and mangrove plant species. An in-depth study of these faunal components in ecological viewpoints may provide pathway towardssome fishes, conservation which are and found management on 7 species of theof mangrove mangrove plants ecosystems. in this ecosystem. But they seem to be non-specific to any Keywords: Epi-Fauna, Habitat, In-Fauna, Mangrove Plants, Odisha Introduction range of species, which occur in high density.