Near2009chap42.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi
Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 (http://www.accessscience.com/) Article by: Boschung, Herbert Department of Biological Sciences, University of Alabama, Tuscaloosa, Alabama. Gardiner, Brian Linnean Society of London, Burlington House, Piccadilly, London, United Kingdom. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.680400 (http://dx.doi.org/10.1036/1097-8542.680400) Content Morphology Euteleostei Bibliography Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi The most recent group of actinopterygians (rayfin fishes), first appearing in the Upper Triassic (Fig. 1). About 26,840 species are contained within the Teleostei, accounting for more than half of all living vertebrates and over 96% of all living fishes. Teleosts comprise 517 families, of which 69 are extinct, leaving 448 extant families; of these, about 43% have no fossil record. See also: Actinopterygii (/content/actinopterygii/009100); Osteichthyes (/content/osteichthyes/478500) Fig. 1 Cladogram showing the relationships of the extant teleosts with the other extant actinopterygians. (J. S. Nelson, Fishes of the World, 4th ed., Wiley, New York, 2006) 1 of 9 10/7/2015 1:07 PM Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 Morphology Much of the evidence for teleost monophyly (evolving from a common ancestral form) and relationships comes from the caudal skeleton and concomitant acquisition of a homocercal tail (upper and lower lobes of the caudal fin are symmetrical). This type of tail primitively results from an ontogenetic fusion of centra (bodies of vertebrae) and the possession of paired bracing bones located bilaterally along the dorsal region of the caudal skeleton, derived ontogenetically from the neural arches (uroneurals) of the ural (tail) centra. -
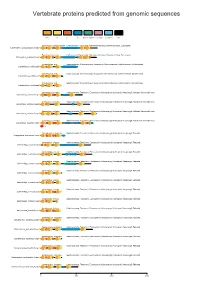
Vertebrate Proteins Predicted from Genomic Sequences
Vertebrate proteins predicted from genomic sequences VWD C8 TIL PTS Mucin2_WxxW F5_F8_type_C FCGBP_N VWC Lethenteron_camtschaticum Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Lethenteron Lethenteron_camtschaticum.0.pep1 Petromyzon_marinus Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Petromyzon Petromyzon_marinus.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep2 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep1 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep2 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.1.pep1 TILa Cynoglossus_semilaevis Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Teleostei; Cynoglossus_semilaevis.1.pep1 -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

Supporting Information
Supporting Information Clarke et al. 10.1073/pnas.1607237113 Major Axes of Shape Variation and Their Anatomical a comparison: the crown teleost vs. stem teleost comparison on Correlates molecular timescales. Here, the larger SL dataset delivers a All relative warp axes that individually account for >5% of the majority of trees where crown teleosts possess significantly variation across the species sampled are displayed in Table S3. higher rates than stem teleosts, whereas the CS and pruned SL Morphospaces derived from the first three axes, containing all datasets find this result in a large minority (Fig. S3). 398 Mesozoic neopterygian species in our shape dataset, are Overall, these results suggest that choice of size metric is presented in Fig. S1. Images of sampled fossil specimens are also relatively unimportant for our dataset, and that the overall size included in Fig. S1 to illustrate the anatomical correlates of and taxonomic samplings of the dataset are more likely to in- shape axes. The positions of major neopterygian clades in mor- fluence subsequent results, despite those factors having a rela- phospace are indicated by different colors in Fig. S2. Major tively small influence here. Nevertheless, choice of metric may be teleost clades are presented in Fig. S2A and major holostean important for other datasets (e.g., different groups of organisms clades in Fig. S2B. or datasets of other biological/nonbiological structures), because RW1 captures 42.53% of the variance, reflecting changes from it is possible to envisage scenarios where the choice of size metric slender-bodied taxa to deep-bodied taxa (Fig. S1 and Table S3). -

History of Fishes - Structural Patterns and Trends in Diversification
History of fishes - Structural Patterns and Trends in Diversification AGNATHANS = Jawless • Class – Pteraspidomorphi • Class – Myxini?? (living) • Class – Cephalaspidomorphi – Osteostraci – Anaspidiformes – Petromyzontiformes (living) Major Groups of Agnathans • 1. Osteostracida 2. Anaspida 3. Pteraspidomorphida • Hagfish and Lamprey = traditionally together in cyclostomata Jaws = GNATHOSTOMES • Gnathostomes: the jawed fishes -good evidence for gnathostome monophyly. • 4 major groups of jawed vertebrates: Extinct Acanthodii and Placodermi (know) Living Chondrichthyes and Osteichthyes • Living Chondrichthyans - usually divided into Selachii or Elasmobranchi (sharks and rays) and Holocephali (chimeroids). • • Living Osteichthyans commonly regarded as forming two major groups ‑ – Actinopterygii – Ray finned fish – Sarcopterygii (coelacanths, lungfish, Tetrapods). • SARCOPTERYGII = Coelacanths + (Dipnoi = Lung-fish) + Rhipidistian (Osteolepimorphi) = Tetrapod Ancestors (Eusthenopteron) Close to tetrapods Lungfish - Dipnoi • Three genera, Africa+Australian+South American ACTINOPTERYGII Bichirs – Cladistia = POLYPTERIFORMES Notable exception = Cladistia – Polypterus (bichirs) - Represented by 10 FW species - tropical Africa and one species - Erpetoichthys calabaricus – reedfish. Highly aberrant Cladistia - numerous uniquely derived features – long, independent evolution: – Strange dorsal finlets, Series spiracular ossicles, Peculiar urohyal bone and parasphenoid • But retain # primitive Actinopterygian features = heavy ganoid scales (external -

The Genetic Factors of Bilaterian Evolution Peter Heger1*, Wen Zheng1†, Anna Rottmann1, Kristen a Panfilio2,3, Thomas Wiehe1
RESEARCH ARTICLE The genetic factors of bilaterian evolution Peter Heger1*, Wen Zheng1†, Anna Rottmann1, Kristen A Panfilio2,3, Thomas Wiehe1 1Institute for Genetics, Cologne Biocenter, University of Cologne, Cologne, Germany; 2Institute for Zoology: Developmental Biology, Cologne Biocenter, University of Cologne, Cologne, Germany; 3School of Life Sciences, University of Warwick, Gibbet Hill Campus, Coventry, United Kingdom Abstract The Cambrian explosion was a unique animal radiation ~540 million years ago that produced the full range of body plans across bilaterians. The genetic mechanisms underlying these events are unknown, leaving a fundamental question in evolutionary biology unanswered. Using large-scale comparative genomics and advanced orthology evaluation techniques, we identified 157 bilaterian-specific genes. They include the entire Nodal pathway, a key regulator of mesoderm development and left-right axis specification; components for nervous system development, including a suite of G-protein-coupled receptors that control physiology and behaviour, the Robo- Slit midline repulsion system, and the neurotrophin signalling system; a high number of zinc finger transcription factors; and novel factors that previously escaped attention. Contradicting the current view, our study reveals that genes with bilaterian origin are robustly associated with key features in extant bilaterians, suggesting a causal relationship. *For correspondence: [email protected] Introduction The taxon Bilateria consists of multicellular animals -

Download Full Article in PDF Format
A new actinopterygian fauna from the latest Cretaceous of Quintanilla la Ojada (Burgos, Spain) Ana BERRETEAGA Universidad del País Vasco/EHU, Facultad de Ciencia y Tecnología, Departamento de Estratigrafía y Paleontología, Apartado 644, SP-48080 Bilbao (Spain) and Universidad de Alcalá de Henares, Facultad de Ciencias, Departamento de Geología, Plaza San Diego s/n, SP-28801 Alcalá de Henares (Spain) [email protected] Francisco José POYATO-ARIZA Universidad Autónoma de Madrid, Departamento de Biología, Unidad de Paleontología, Cantoblanco, SP-28049 Madrid (Spain ) [email protected] Xabier PEREDA-SUBERBIOLA Universidad del País Vasco/EHU, Facultad de Ciencia y Tecnología, Departamento de Estratigrafía y Paleontología, Apartado 644, SP-48080 Bilbao (Spain) [email protected] Berreteaga A., Poyato-Ariza F. J. & Pereda-Suberbiola X. 2011. — A new actinopterygian fauna from the latest Cretaceous of Quintanilla la Ojada (Burgos, Spain). Geodiversitas 33 (2): 285-301. DOI: 10.5252/g2011n2a6. ABSTRACT We describe a new actinopterygian fauna from the uppermost Cretaceous of Quintanilla la Ojada (Burgos, Spain), in the Villarcayo Sinclynorium of the Basque-Cantabrian Region. It consists mostly of isolated teeth of pycnodon- tiforms (cf. Anomoeodus sp., Pycnodontoidea indet.), amiiforms (cf. Amiidae indet.) and teleosteans (elopiforms: Phyllodontinae indet., Paralbulinae indet.; KEY WORDS aulopiforms: Enchodontidae indet., plus fragmentary fi n spines of Acantho- Osteichthyes, Pycnodontiformes, morpha indet.). Paralbulinae teeth are the most abundant elements in the fossil Amiiformes, assemblage. All the remains are disarticulated and show intense post-mortem Elopiformes, Aulopiformes, abrasion. Th e fossil association has been found in dolomite sandstones that are Acanthomorpha, laterally correlated with the Valdenoceda Formation (Lower to basal Upper Maastrichtian, Maastrichtian) of the Castilian Ramp. -

Fine Structure of the Gas Bladder of Alligator Gar, Atractosteus Spatula Ahmad Omar-Ali1, Wes Baumgartner2, Peter J
www.symbiosisonline.org Symbiosis www.symbiosisonlinepublishing.com International Journal of Scientific Research in Environmental Science and Toxicology Research Article Open Access Fine Structure of the Gas Bladder of Alligator Gar, Atractosteus spatula Ahmad Omar-Ali1, Wes Baumgartner2, Peter J. Allen3, Lora Petrie-Hanson1* 1Department of Basic Sciences, College of Veterinary Medicine, Mississippi State University, Mississippi State, MS 39762, USA 2Department of Pathobiology and Population Medicine, College of Veterinary Medicine, Mississippi State University, Mississippi State, MS 39762, USA 3Department of Wildlife, Fisheries and Aquaculture, College of Forest Resources, Mississippi State University, Mississippi State, MS 39762, USA Received: 1 November, 2016; Accepted: 2 December, 2016 ; Published: 12 December, 2016 *Corresponding author: Lora Petrie-Hanson, Associate Professor, College of Veterinary Medicine, 240 Wise Center Drive PO Box 6100, Mississippi State, MS 39762, USA, Tel: +1-(601)-325-1291; Fax: +1-(662)325-1031; E-mail: [email protected] Lepisosteidae includes the genera Atractosteus and Lepisosteus. Abstract Atractosteus includes A. spatula (alligator gar), A. tristoechus Anthropogenic factors seriously affect water quality and (Cuban gar), and A. tropicus (tropical gar), while Lepisosteus includes L. oculatus (spotted gar), L. osseus (long nose gar), L. in the Mississippi River and the coastal estuaries. Alligator gar platostomus (short nose gar), and L. platyrhincus (Florida gar) (adversely affect fish )populations. inhabits these Agricultural waters andrun-off is impactedaccumulates by Atractosteus spatula [2,6,7]. Atractosteus are distinguishable from Lepisosteus by agricultural pollution, petrochemical contaminants and oil spills. shorter, more numerous gill rakers and a more prominent accessory organ. The gas bladder, or Air Breathing Organ (ABO) of second row of teeth in the upper jaw [2]. -

Body-Shape Diversity in Triassic–Early Cretaceous Neopterygian fishes: Sustained Holostean Disparity and Predominantly Gradual Increases in Teleost Phenotypic Variety
Body-shape diversity in Triassic–Early Cretaceous neopterygian fishes: sustained holostean disparity and predominantly gradual increases in teleost phenotypic variety John T. Clarke and Matt Friedman Comprising Holostei and Teleostei, the ~32,000 species of neopterygian fishes are anatomically disparate and represent the dominant group of aquatic vertebrates today. However, the pattern by which teleosts rose to represent almost all of this diversity, while their holostean sister-group dwindled to eight extant species and two broad morphologies, is poorly constrained. A geometric morphometric approach was taken to generate a morphospace from more than 400 fossil taxa, representing almost all articulated neopterygian taxa known from the first 150 million years— roughly 60%—of their history (Triassic‒Early Cretaceous). Patterns of morphospace occupancy and disparity are examined to: (1) assess evidence for a phenotypically “dominant” holostean phase; (2) evaluate whether expansions in teleost phenotypic variety are predominantly abrupt or gradual, including assessment of whether early apomorphy-defined teleosts are as morphologically conservative as typically assumed; and (3) compare diversification in crown and stem teleosts. The systematic affinities of dapediiforms and pycnodontiforms, two extinct neopterygian clades of uncertain phylogenetic placement, significantly impact patterns of morphological diversification. For instance, alternative placements dictate whether or not holosteans possessed statistically higher disparity than teleosts in the Late Triassic and Jurassic. Despite this ambiguity, all scenarios agree that holosteans do not exhibit a decline in disparity during the Early Triassic‒Early Cretaceous interval, but instead maintain their Toarcian‒Callovian variety until the end of the Early Cretaceous without substantial further expansions. After a conservative Induan‒Carnian phase, teleosts colonize (and persistently occupy) novel regions of morphospace in a predominantly gradual manner until the Hauterivian, after which expansions are rare. -

Master List of Fishes
FISHES OF THE FRESHWATER POTOMAC Compiled by Jim Cummins, The Interstate Commission on the Potomac River Basin Always DRAFT - Version 02/21/2013 The following list of one-hundred and eighteen fish species known to be present in the freshwater portions of the Potomac River basin. Included, but not numbered, are fish that once were in the Potomac but are no longer are present; eight extirpated fish species (only one of which, the log perch, was perhaps a native to the Potomac) and three with uncertain presences. The list was originally (1995) compiled through a combination of personal field experience, a search of the literature, and input from regional fisheries biologists Ed Enamait (MD), Gerald Lewis (WV), Ed Stienkoenig (VA), and Jon Siemiens (DC). However, I attempt to keep the list updated when new information becomes available, thus the list is always draft. The distribution of these fishes within the Potomac is highly variable. Many are year-round residents and are fairly wide-spread, while some, such as the torrent shiner, are only found in very limited habitats/areas. Eleven are migratory species which typically come into the river system to spawn, and nine represent occasional visitors in freshwater-tidal areas. The native or introduced status of most of these species are generally accepted, but for some species this status is an object of continued researched and therefore caution should be used in interpreting this designation, especially when noted with a “?” mark. Of the 118 species currently found in the river, approximately 80 (68%) are considered native, 23 (19%) are considered introduced, and the rest (15, or 13%) are uncertain in origin. -

A Phylogenomic Perspective on the Radiation of Ray-Finned Fishes Based Upon Targeted Sequencing of Ultraconserved Elements
1 A phylogenomic perspective on the radiation of 2 ray-finned fishes based upon targeted sequencing of 3 ultraconserved elements 1;2;∗ 1;2 1 1 4 Michael E. Alfaro , Brant C. Faircloth , Laurie Sorenson , Francesco Santini 1 5 Department of Ecology and Evolutionary Biology, University of California, Los Angeles, CA, USA 2 6 These authors contributed equally to this work 7 ∗ E-mail: [email protected] arXiv:1210.0120v1 [q-bio.PE] 29 Sep 2012 1 8 Summary 9 Ray-finned fishes constitute the dominant radiation of vertebrates with over 30,000 species. 10 Although molecular phylogenetics has begun to disentangle major evolutionary relationships 11 within this vast section of the Tree of Life, there is no widely available approach for effi- 12 ciently collecting phylogenomic data within fishes, leaving much of the enormous potential 13 of massively parallel sequencing technologies for resolving major radiations in ray-finned 14 fishes unrealized. Here, we provide a genomic perspective on longstanding questions regard- 15 ing the diversification of major groups of ray-finned fishes through targeted enrichment of 16 ultraconserved nuclear DNA elements (UCEs) and their flanking sequence. Our workflow 17 efficiently and economically generates data sets that are orders of magnitude larger than 18 those produced by traditional approaches and is well-suited to working with museum speci- 19 mens. Analysis of the UCE data set recovers a well-supported phylogeny at both shallow and 20 deep time-scales that supports a monophyletic relationship between Amia and Lepisosteus 21 (Holostei) and reveals elopomorphs and then osteoglossomorphs to be the earliest diverging 22 teleost lineages.