The Evolutionary Tree of Life
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Closest Unicellular Relatives of Animals
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology, Vol. 12, 1773–1778, October 15, 2002, 2002 Elsevier Science Ltd. All rights reserved. PII S0960-9822(02)01187-9 The Closest Unicellular Relatives of Animals B.F. Lang,1,2 C. O’Kelly,1,3 T. Nerad,4 M.W. Gray,1,5 Results and Discussion and G. Burger1,2,6 1The Canadian Institute for Advanced Research The evolution of the Metazoa from single-celled protists Program in Evolutionary Biology is an issue that has intrigued biologists for more than a 2 De´ partement de Biochimie century. Early morphological and more recent ultra- Universite´ de Montre´ al structural and molecular studies have converged in sup- Succursale Centre-Ville porting the now widely accepted view that animals are Montre´ al, Que´ bec H3C 3J7 related to Fungi, choanoflagellates, and ichthyosporean Canada protists. However, controversy persists as to the spe- 3 Bigelow Laboratory for Ocean Sciences cific evolutionary relationships among these major P.O. Box 475 groups. This uncertainty is reflected in the plethora of 180 McKown Point Road published molecular phylogenies that propose virtually West Boothbay Harbor, Maine 04575 all of the possible alternative tree topologies involving 4 American Type Culture Collection Choanoflagellata, Fungi, Ichthyosporea, and Metazoa. 10801 University Boulevard For example, a monophyletic MetazoaϩChoanoflagel- Manassas, Virginia 20110 lata group has been suggested on the basis of small 5 Department of Biochemistry subunit (SSU) rDNA sequences [1, 6, 7]. Other studies and Molecular Biology using the same sequences have allied Choanoflagellata Dalhousie University with the Fungi [8], placed Choanoflagellata prior to the Halifax, Nova Scotia B3H 4H7 divergence of animals and Fungi [9], or even placed Canada them prior to the divergence of green algae and land plants [10]. -

The Phagotrophic Origin of Eukaryotes and Phylogenetic Classification Of
International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 DOI: 10.1099/ijs.0.02058-0 The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa Department of Zoology, T. Cavalier-Smith University of Oxford, South Parks Road, Oxford OX1 3PS, UK Tel: j44 1865 281065. Fax: j44 1865 281310. e-mail: tom.cavalier-smith!zoo.ox.ac.uk Eukaryotes and archaebacteria form the clade neomura and are sisters, as shown decisively by genes fragmented only in archaebacteria and by many sequence trees. This sisterhood refutes all theories that eukaryotes originated by merging an archaebacterium and an α-proteobacterium, which also fail to account for numerous features shared specifically by eukaryotes and actinobacteria. I revise the phagotrophy theory of eukaryote origins by arguing that the essentially autogenous origins of most eukaryotic cell properties (phagotrophy, endomembrane system including peroxisomes, cytoskeleton, nucleus, mitosis and sex) partially overlapped and were synergistic with the symbiogenetic origin of mitochondria from an α-proteobacterium. These radical innovations occurred in a derivative of the neomuran common ancestor, which itself had evolved immediately prior to the divergence of eukaryotes and archaebacteria by drastic alterations to its eubacterial ancestor, an actinobacterial posibacterium able to make sterols, by replacing murein peptidoglycan by N-linked glycoproteins and a multitude of other shared neomuran novelties. The conversion of the rigid neomuran wall into a flexible surface coat and the associated origin of phagotrophy were instrumental in the evolution of the endomembrane system, cytoskeleton, nuclear organization and division and sexual life-cycles. Cilia evolved not by symbiogenesis but by autogenous specialization of the cytoskeleton. -

Supplementary Material Parameter Unit Average ± Std NO3 + NO2 Nm
Supplementary Material Table S1. Chemical and biological properties of the NRS water used in the experiment (before amendments). Parameter Unit Average ± std NO3 + NO2 nM 140 ± 13 PO4 nM 8 ± 1 DOC μM 74 ± 1 Fe nM 8.5 ± 1.8 Zn nM 8.7 ± 2.1 Cu nM 1.4 ± 0.9 Bacterial abundance Cells × 104/mL 350 ± 15 Bacterial production μg C L−1 h−1 1.41 ± 0.08 Primary production μg C L−1 h−1 0.60 ± 0.01 β-Gl nM L−1 h−1 1.42 ± 0.07 APA nM L−1 h−1 5.58 ± 0.17 AMA nM L−1·h−1 2.60 ± 0.09 Chl-a μg/L 0.28 ± 0.01 Prochlorococcus cells × 104/mL 1.49 ± 02 Synechococcus cells × 104/mL 5.14 ± 1.04 pico-eukaryot cells × 103/mL 1.58 × 0.1 Table S2. Nutrients and trace metals concentrations added from the aerosols to each mesocosm. Variable Unit Average ± std NO3 + NO2 nM 48 ± 2 PO4 nM 2.4 ± 1 DOC μM 165 ± 2 Fe nM 2.6 ± 1.5 Zn nM 6.7 ± 2.5 Cu nM 0.6 ± 0.2 Atmosphere 2019, 10, 358; doi:10.3390/atmos10070358 www.mdpi.com/journal/atmosphere Atmosphere 2019, 10, 358 2 of 6 Table S3. ANOVA test results between control, ‘UV-treated’ and ‘live-dust’ treatments at 20 h or 44 h, with significantly different values shown in bold. ANOVA df Sum Sq Mean Sq F Value p-value Chl-a 20 H 2, 6 0.03, 0.02 0.02, 0 4.52 0.0634 44 H 2, 6 0.02, 0 0.01, 0 23.13 0.002 Synechococcus Abundance 20 H 2, 7 8.23 × 107, 4.11 × 107 4.11 × 107, 4.51 × 107 0.91 0.4509 44 H 2, 7 5.31 × 108, 6.97 × 107 2.65 × 108, 1.16 × 107 22.84 0.0016 Prochlorococcus Abundance 20 H 2, 8 4.22 × 107, 2.11 × 107 2.11 × 107, 2.71 × 106 7.77 0.0216 44 H 2, 8 9.02 × 107, 1.47 × 107 4.51 × 107, 2.45 × 106 18.38 0.0028 Pico-eukaryote -
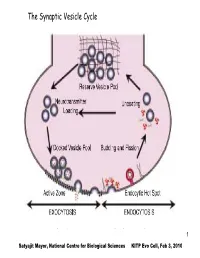
The Synaptic Vesicle Cycle
The Synaptic Vesicle Cycle 1 Satyajit Mayor, National Centre for Biological Sciences KITP Evo Cell, Feb 3, 2010 The Eukaryotic Commandments: T. Cavalier-Smith (i)origin of the endomembrane system (ER, Golgi and lysosomes) and coated- vesicle budding and fusion, including endocytosis and exocytosis; (ii) origin of the cytoskeleton, centrioles, cilia and associated molecular motors; (iii) origin of the nucleus, nuclear pore complex and trans-envelope protein and RNA transport; (iv) origin of linear chromosomes with plural replicons, centromeres and telomeres; (v) origin of novel cell-cycle controls and mitotic segregation; (vi) origin of sex (syngamy, nuclear fusion and meiosis); (vii) origin of peroxisomes; (ix) origin of mitochondria; (viii) novel patterns of rRNAprocessing using small nucleolar-ribonucleoproteins (snoRNPs); (x) origin of spliceosomal introns. 2 EM of Chemical synapse mitochondria Active Zone Figure 5.3, Bear, 2001 3 T. Cavalier-Smith International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 Fig. 1. The bacterial origins of eukaryotes as a two-stage process. I phase: The ancestors of eukaryotes, the stem neomura, are shared with archaebacteria and evolved during the neomuran revolution, in which N-linked glycoproteins replaced murein peptidoglycan and 18 other suites of characters changed radically through adaptation of an ancestral actinobacterium to thermophily, as discussed in detail by Cavalier- Smith (2002a). 4 T. Cavalier-Smith International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 Fig. 1. The bacterial origins of eukaryotes as a two-stage process. II phase: In the next phase, archaebacteria and eukaryotes diverged dramatically. Archaebacteria retained the wall and therefore their general bacterial cell and genetic organization, but became adapted to even hotter and more acidic environments by substituting prenyl ether lipids for the ancestral acyl esters and making new acid- resistant flagellar shafts (Cavalier-Smith, 2002a). -

Predatory Flagellates – the New Recently Discovered Deep Branches of the Eukaryotic Tree and Their Evolutionary and Ecological Significance
Protistology 14 (1), 15–22 (2020) Protistology Predatory flagellates – the new recently discovered deep branches of the eukaryotic tree and their evolutionary and ecological significance Denis V. Tikhonenkov Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok, 152742, Russia | Submitted March 20, 2020 | Accepted April 6, 2020 | Summary Predatory protists are poorly studied, although they are often representing important deep-branching evolutionary lineages and new eukaryotic supergroups. This short review/opinion paper is inspired by the recent discoveries of various predatory flagellates, which form sister groups of the giant eukaryotic clusters on phylogenetic trees, and illustrate an ancestral state of one or another supergroup of eukaryotes. Here we discuss their evolutionary and ecological relevance and show that the study of such protists may be essential in addressing previously puzzling evolutionary problems, such as the origin of multicellular animals, the plastid spread trajectory, origins of photosynthesis and parasitism, evolution of mitochondrial genomes. Key words: evolution of eukaryotes, heterotrophic flagellates, mitochondrial genome, origin of animals, photosynthesis, predatory protists, tree of life Predatory flagellates and diversity of eu- of the hidden diversity of protists (Moon-van der karyotes Staay et al., 2000; López-García et al., 2001; Edg- comb et al., 2002; Massana et al., 2004; Richards The well-studied multicellular animals, plants and Bass, 2005; Tarbe et al., 2011; de Vargas et al., and fungi immediately come to mind when we hear 2015). In particular, several prevailing and very abun- the term “eukaryotes”. However, these groups of dant ribogroups such as MALV, MAST, MAOP, organisms represent a minority in the real diversity MAFO (marine alveolates, stramenopiles, opistho- of evolutionary lineages of eukaryotes. -

S41467-021-25308-W.Pdf
ARTICLE https://doi.org/10.1038/s41467-021-25308-w OPEN Phylogenomics of a new fungal phylum reveals multiple waves of reductive evolution across Holomycota ✉ ✉ Luis Javier Galindo 1 , Purificación López-García 1, Guifré Torruella1, Sergey Karpov2,3 & David Moreira 1 Compared to multicellular fungi and unicellular yeasts, unicellular fungi with free-living fla- gellated stages (zoospores) remain poorly known and their phylogenetic position is often 1234567890():,; unresolved. Recently, rRNA gene phylogenetic analyses of two atypical parasitic fungi with amoeboid zoospores and long kinetosomes, the sanchytrids Amoeboradix gromovi and San- chytrium tribonematis, showed that they formed a monophyletic group without close affinity with known fungal clades. Here, we sequence single-cell genomes for both species to assess their phylogenetic position and evolution. Phylogenomic analyses using different protein datasets and a comprehensive taxon sampling result in an almost fully-resolved fungal tree, with Chytridiomycota as sister to all other fungi, and sanchytrids forming a well-supported, fast-evolving clade sister to Blastocladiomycota. Comparative genomic analyses across fungi and their allies (Holomycota) reveal an atypically reduced metabolic repertoire for sanchy- trids. We infer three main independent flagellum losses from the distribution of over 60 flagellum-specific proteins across Holomycota. Based on sanchytrids’ phylogenetic position and unique traits, we propose the designation of a novel phylum, Sanchytriomycota. In addition, our results indicate that most of the hyphal morphogenesis gene repertoire of multicellular fungi had already evolved in early holomycotan lineages. 1 Ecologie Systématique Evolution, CNRS, Université Paris-Saclay, AgroParisTech, Orsay, France. 2 Zoological Institute, Russian Academy of Sciences, St. ✉ Petersburg, Russia. 3 St. -

Origin of the Cell Nucleus, Mitosis and Sex: Roles of Intracellular Coevolution Thomas Cavalier-Smith*
Cavalier-Smith Biology Direct 2010, 5:7 http://www.biology-direct.com/content/5/1/7 RESEARCH Open Access Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution Thomas Cavalier-Smith* Abstract Background: The transition from prokaryotes to eukaryotes was the most radical change in cell organisation since life began, with the largest ever burst of gene duplication and novelty. According to the coevolutionary theory of eukaryote origins, the fundamental innovations were the concerted origins of the endomembrane system and cytoskeleton, subsequently recruited to form the cell nucleus and coevolving mitotic apparatus, with numerous genetic eukaryotic novelties inevitable consequences of this compartmentation and novel DNA segregation mechanism. Physical and mutational mechanisms of origin of the nucleus are seldom considered beyond the long- standing assumption that it involved wrapping pre-existing endomembranes around chromatin. Discussions on the origin of sex typically overlook its association with protozoan entry into dormant walled cysts and the likely simultaneous coevolutionary, not sequential, origin of mitosis and meiosis. Results: I elucidate nuclear and mitotic coevolution, explaining the origins of dicer and small centromeric RNAs for positionally controlling centromeric heterochromatin, and how 27 major features of the cell nucleus evolved in four logical stages, making both mechanisms and selective advantages explicit: two initial stages (origin of 30 nm chromatin fibres, enabling DNA compaction; and firmer attachment of endomembranes to heterochromatin) protected DNA and nascent RNA from shearing by novel molecular motors mediating vesicle transport, division, and cytoplasmic motility. Then octagonal nuclear pore complexes (NPCs) arguably evolved from COPII coated vesicle proteins trapped in clumps by Ran GTPase-mediated cisternal fusion that generated the fenestrated nuclear envelope, preventing lethal complete cisternal fusion, and allowing passive protein and RNA exchange. -

Protists – a Textbook Example for a Paraphyletic Taxon
ARTICLE IN PRESS Organisms, Diversity & Evolution 7 (2007) 166–172 www.elsevier.de/ode Protists – A textbook example for a paraphyletic taxon$ Martin Schlegela,Ã, Norbert Hu¨lsmannb aInstitute for Biology II, University of Leipzig, Talstraße 33, 04103 Leipzig, Germany bFree University of Berlin, Institute of Biology/Zoology, Working group Protozoology, Ko¨nigin-Luise-Straße 1-3, 14195 Berlin, Germany Received 7 September 2004; accepted 21 November 2006 Abstract Protists constitute a paraphyletic taxon since the latter is based on the plesiomorphic character of unicellularity and does not contain all descendants of the stem species. Multicellularity evolved several times independently in metazoans, higher fungi, heterokonts, red and green algae. Various hypotheses have been developed on the evolution and nature of the eukaryotic cell, considering the accumulating data on the chimeric nature of the eukaryote genome. Subsequent evolution of the protists was further complicated by primary, secondary, and even tertiary intertaxonic recombinations. However, multi-gene sequence comparisons and structural data point to a managable number of such events. Several putative monophyletic lineages and a gross picture of eukaryote phylogeny are emerging on the basis of those data. The Chromalveolata comprise Chromista and Alveolata (Dinoflagellata, Apicomplexa, Ciliophora, Perkinsozoa, and Haplospora). Major lineages of the former ‘amoebae’ group within the Heterolobosa, Cercozoa, and Amoebozoa. Cercozoa, including filose testate amoebae, chlorarachnids, and plasmodiophoreans seem to be affiliated with foraminiferans. Amoebozoa consistently form the sister group of the Opisthokonta (including fungi, and with choanoflagellates as sister group of metazoans). A clade of ‘plants’ comprises glaucocystophytes, red algae, green algae, and land vascular plants. The controversial debate on the root of the eukaryote tree has been accelerated by the interpretation of gene fusions as apomorphic characters. -

Microbial Eukaryotes in Oil Sands Environments: Heterotrophs in the Spotlight
microorganisms Review Microbial Eukaryotes in Oil Sands Environments: Heterotrophs in the Spotlight Elisabeth Richardson 1,* and Joel B. Dacks 2,* 1 Department of Biological Sciences, University of Alberta, Edmonton, AB T6G 2E9, Canada 2 Division of Infectious Disease, Department of Medicine, University of Alberta, Edmonton, AB T6G 2G3, Canada * Correspondence: [email protected] (E.R.); [email protected] (J.B.D.); Tel.: +1-780-248-1493 (J.B.D.) Received: 6 May 2019; Accepted: 14 June 2019; Published: 19 June 2019 Abstract: Hydrocarbon extraction and exploitation is a global, trillion-dollar industry. However, for decades it has also been known that fossil fuel usage is environmentally detrimental; the burning of hydrocarbons results in climate change, and environmental damage during extraction and transport can also occur. Substantial global efforts into mitigating this environmental disruption are underway. The global petroleum industry is moving more and more into exploiting unconventional oil reserves, such as oil sands and shale oil. The Albertan oil sands are one example of unconventional oil reserves; this mixture of sand and heavy bitumen lying under the boreal forest of Northern Alberta represent one of the world’s largest hydrocarbon reserves, but extraction also requires the disturbance of a delicate northern ecosystem. Considerable effort is being made by various stakeholders to mitigate environmental impact and reclaim anthropogenically disturbed environments associated with oil sand extraction. In this review, we discuss the eukaryotic microbial communities associated with the boreal ecosystem and how this is affected by hydrocarbon extraction, with a particular emphasis on the reclamation of tailings ponds, where oil sands extraction waste is stored. -

Tracing Evolutionary Ages of Cancer-Driving Sites by Cancer
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.09.940528; this version posted February 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Tracing Evolutionary Ages of Cancer-Driving Sites by Cancer Somatic Mutations Xun Gu*1, Zhan Zou2, Jingwen Yang3,4 1Department of Genetics, Development and Cell Biology, Iowa State University, Ames, IA 50011, USA. 2College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 301158, China 3School of Life Sciences, Fudan University, Shanghai 200433, China. 4Human Phenome Institute, Fudan University, Shanghai 200433, China. *Corresponding author. E-mail: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.09.940528; this version posted February 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Evolutionary understanding of cancer genes may provide insights on the nature and evolution of complex life and the origin of multicellularity. In this study, we focus on the evolutionary ages of cancer-driving sites, and try to explore to what extent the amino acids of cancer-driving sites can be traced back to the most recent common ancestor (MRCA) of the gene. According to gene phylostraigraphy analysis, we use the definition of gene age (tg) by the most ancient phylogenetic position that can be traced back, in most cases based on the large-scale homology search of protein sequences. -

Molecular Phylogeny of Choanoflagellates, the Sister Group to Metazoa
Molecular phylogeny of choanoflagellates, the sister group to Metazoa M. Carr*†, B. S. C. Leadbeater*‡, R. Hassan‡§, M. Nelson†, and S. L. Baldauf†¶ʈ †Department of Biology, University of York, Heslington, York, YO10 5YW, United Kingdom; and ‡School of Biosciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, United Kingdom Edited by Andrew H. Knoll, Harvard University, Cambridge, MA, and approved August 28, 2008 (received for review February 28, 2008) Choanoflagellates are single-celled aquatic flagellates with a unique family. Members of the Acanthoecidae family (Norris 1965) are morphology consisting of a cell with a single flagellum surrounded by characterized by the most distinct periplast morphology. This a ‘‘collar’’ of microvilli. They have long interested evolutionary biol- consists of a complex basket-like lorica constructed in a precise and ogists because of their striking resemblance to the collared cells highly reproducible manner from ribs (costae) composed of rod- (choanocytes) of sponges. Molecular phylogeny has confirmed a close shaped silica strips (Fig. 1 E and F) (13). The Acanthoecidae family relationship between choanoflagellates and Metazoa, and the first is further subdivided into nudiform (Fig. 1E) and tectiform (Fig. 1F) choanoflagellate genome sequence has recently been published. species, based on the morphology of the lorica, the stage in the cell However, molecular phylogenetic studies within choanoflagellates cycle when the silica strips are produced, the location at which the are still extremely limited. Thus, little is known about choanoflagel- strips are stored, and the mode of cell division [supporting infor- late evolution or the exact nature of the relationship between mation (SI) Text] (14). -

Multigene Phylogeny of Choanozoa and the Origin of Animals
Multigene Phylogeny of Choanozoa and the Origin of Animals Kamran Shalchian-Tabrizi1*, Marianne A. Minge2, Mari Espelund2, Russell Orr1, Torgeir Ruden3, Kjetill S. Jakobsen2, Thomas Cavalier-Smith4 1 Microbial Evolution Research Group, Department of Biology, University of Oslo, Oslo, Norway, 2 Centre for Ecological and Evolutionary Synthesis, University of Oslo, Oslo, Norway, 3 Scientific Computer Group, Center for Information Technology Services, University of Oslo, Oslo, Norway, 4 Department of Zoology, University of Oxford, Oxford, United Kingdom Abstract Animals are evolutionarily related to fungi and to the predominantly unicellular protozoan phylum Choanozoa, together known as opisthokonts. To establish the sequence of events when animals evolved from unicellular ancestors, and understand those key evolutionary transitions, we need to establish which choanozoans are most closely related to animals and also the evolutionary position of each choanozoan group within the opisthokont phylogenetic tree. Here we focus on Ministeria vibrans, a minute bacteria-eating cell with slender radiating tentacles. Single-gene trees suggested that it is either the closest unicellular relative of animals or else sister to choanoflagellates, traditionally considered likely animal ancestors. Sequencing thousands of Ministeria protein genes now reveals about 14 with domains of key significance for animal cell biology, including several previously unknown from deeply diverging Choanozoa, e.g. domains involved in hedgehog, Notch and tyrosine kinase signaling