Transcription Factor Evolution in Eukaryotes and the Assembly of the Regulatory Toolkit in Multicellular Lineages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calcium Affects Polyphosphate and Lipid Accumulation in Mucoromycota Fungi
Journal of Fungi Article Calcium Affects Polyphosphate and Lipid Accumulation in Mucoromycota Fungi Simona Dzurendova 1,*, Boris Zimmermann 1 , Achim Kohler 1, Kasper Reitzel 2 , Ulla Gro Nielsen 3 , Benjamin Xavier Dupuy--Galet 1 , Shaun Leivers 4 , Svein Jarle Horn 4 and Volha Shapaval 1 1 Faculty of Science and Technology, Norwegian University of Life Sciences, Drøbakveien 31, 1433 Ås, Norway; [email protected] (B.Z.); [email protected] (A.K.); [email protected] (B.X.D.–G.); [email protected] (V.S.) 2 Department of Biology, University of Southern Denmark, Campusvej 55, DK-5230 Odense M, Denmark; [email protected] 3 Department of Physics, Chemistry and Pharmacy, University of Southern Denmark, Campusvej 55, DK-5230 Odense M, Denmark; [email protected] 4 Faculty of Chemistry, Biotechnology and Food Science, Norwegian University of Life Sciences, Christian Magnus Falsens vei 1, 1433 Ås, Norway; [email protected] (S.L.); [email protected] (S.J.H.) * Correspondence: [email protected] or [email protected] Abstract: Calcium controls important processes in fungal metabolism, such as hyphae growth, cell wall synthesis, and stress tolerance. Recently, it was reported that calcium affects polyphosphate and lipid accumulation in fungi. The purpose of this study was to assess the effect of calcium on the accumulation of lipids and polyphosphate for six oleaginous Mucoromycota fungi grown under different phosphorus/pH conditions. A Duetz microtiter plate system (Duetz MTPS) was used for the cultivation. The compositional profile of the microbial biomass was recorded using Fourier-transform infrared spectroscopy, the high throughput screening extension (FTIR-HTS). -

Characterization of Two Undescribed Mucoralean Species with Specific
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 26 March 2018 doi:10.20944/preprints201803.0204.v1 1 Article 2 Characterization of Two Undescribed Mucoralean 3 Species with Specific Habitats in Korea 4 Seo Hee Lee, Thuong T. T. Nguyen and Hyang Burm Lee* 5 Division of Food Technology, Biotechnology and Agrochemistry, College of Agriculture and Life Sciences, 6 Chonnam National University, Gwangju 61186, Korea; [email protected] (S.H.L.); 7 [email protected] (T.T.T.N.) 8 * Correspondence: [email protected]; Tel.: +82-(0)62-530-2136 9 10 Abstract: The order Mucorales, the largest in number of species within the Mucoromycotina, 11 comprises typically fast-growing saprotrophic fungi. During a study of the fungal diversity of 12 undiscovered taxa in Korea, two mucoralean strains, CNUFC-GWD3-9 and CNUFC-EGF1-4, were 13 isolated from specific habitats including freshwater and fecal samples, respectively, in Korea. The 14 strains were analyzed both for morphology and phylogeny based on the internal transcribed 15 spacer (ITS) and large subunit (LSU) of 28S ribosomal DNA regions. On the basis of their 16 morphological characteristics and sequence analyses, isolates CNUFC-GWD3-9 and CNUFC- 17 EGF1-4 were confirmed to be Gilbertella persicaria and Pilobolus crystallinus, respectively.To the 18 best of our knowledge, there are no published literature records of these two genera in Korea. 19 Keywords: Gilbertella persicaria; Pilobolus crystallinus; mucoralean fungi; phylogeny; morphology; 20 undiscovered taxa 21 22 1. Introduction 23 Previously, taxa of the former phylum Zygomycota were distributed among the phylum 24 Glomeromycota and four subphyla incertae sedis, including Mucoromycotina, Kickxellomycotina, 25 Zoopagomycotina, and Entomophthoromycotina [1]. -

Supplementary Table S2: New Taxonomic Assignment of Sequences of Basal Fungal Lineages
Supplementary Table S2: New taxonomic assignment of sequences of basal fungal lineages. Fungal sequences were subjected to BLAST-N analysis and checked for their taxonomic placement in the eukaryotic guide-tree of the SILVA release 111. Sequences were classified depending on combined results from the methods mentioned above as well as literature searches. Accession Name New classification Clustering of the sequence in the Best BLAST-N hit number based on combined results eukaryotic guide tree of SILVA Name Accession number E.value Identity AB191431 Uncultured fungus Chytridiomycota Chytridiomycota Basidiobolus haptosporus AF113413.1 0.0 91 AB191432 Unculltured eukaryote Blastocladiomycota Blastocladiomycota Rhizophlyctis rosea NG_017175.1 0.0 91 AB252775 Uncultured eukaryote Chytridiomycota Chytridiomycota Blastocladiales sp. EF565163.1 0.0 91 AB252776 Uncultured eukaryote Fungi Nucletmycea_Fonticula Rhizophydium sp. AF164270.2 0.0 87 AB252777 Uncultured eukaryote Chytridiomycota Chytridiomycota Basidiobolus haptosporus AF113413.1 0.0 91 AB275063 Uncultured fungus Chytridiomycota Chytridiomycota Catenomyces sp. AY635830.1 0.0 90 AB275064 Uncultured fungus Chytridiomycota Chytridiomycota Endogone lactiflua DQ536471.1 0.0 91 AB433328 Nuclearia thermophila Nuclearia Nucletmycea_Nuclearia Nuclearia thermophila AB433328.1 0.0 100 AB468592 Uncultured fungus Basal clone group I Chytridiomycota Physoderma dulichii DQ536472.1 0.0 90 AB468593 Uncultured fungus Basal clone group I Chytridiomycota Physoderma dulichii DQ536472.1 0.0 91 AB468594 Uncultured -

Downloaded (Additional File 1, Table S4)
Phylogenomics supports microsporidia as the earliest diverging clade of sequenced fungi Capella-Gutiérrez et al. Capella-Gutiérrez et al. BMC Biology 2012, 10:47 http://www.biomedcentral.com/1741-7007/10/47 (31 May 2012) Capella-Gutiérrez et al. BMC Biology 2012, 10:47 http://www.biomedcentral.com/1741-7007/10/47 RESEARCHARTICLE Open Access Phylogenomics supports microsporidia as the earliest diverging clade of sequenced fungi Salvador Capella-Gutiérrez, Marina Marcet-Houben and Toni Gabaldón* Abstract Background: Microsporidia is one of the taxa that have experienced the most dramatic taxonomic reclassifications. Once thought to be among the earliest diverging eukaryotes, the fungal nature of this group of intracellular pathogens is now widely accepted. However, the specific position of microsporidia within the fungal tree of life is still debated. Due to the presence of accelerated evolutionary rates, phylogenetic analyses involving microsporidia are prone to methodological artifacts, such as long-branch attraction, especially when taxon sampling is limited. Results: Here we exploit the recent availability of six complete microsporidian genomes to re-assess the long- standing question of their phylogenetic position. We show that microsporidians have a similar low level of conservation of gene neighborhood with other groups of fungi when controlling for the confounding effects of recent segmental duplications. A combined analysis of thousands of gene trees supports a topology in which microsporidia is a sister group to all other sequenced fungi. Moreover, this topology received increased support when less informative trees were discarded. This position of microsporidia was also strongly supported based on the combined analysis of 53 concatenated genes, and was robust to filters controlling for rate heterogeneity, compositional bias, long branch attraction and heterotachy. -

The Closest Unicellular Relatives of Animals
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology, Vol. 12, 1773–1778, October 15, 2002, 2002 Elsevier Science Ltd. All rights reserved. PII S0960-9822(02)01187-9 The Closest Unicellular Relatives of Animals B.F. Lang,1,2 C. O’Kelly,1,3 T. Nerad,4 M.W. Gray,1,5 Results and Discussion and G. Burger1,2,6 1The Canadian Institute for Advanced Research The evolution of the Metazoa from single-celled protists Program in Evolutionary Biology is an issue that has intrigued biologists for more than a 2 De´ partement de Biochimie century. Early morphological and more recent ultra- Universite´ de Montre´ al structural and molecular studies have converged in sup- Succursale Centre-Ville porting the now widely accepted view that animals are Montre´ al, Que´ bec H3C 3J7 related to Fungi, choanoflagellates, and ichthyosporean Canada protists. However, controversy persists as to the spe- 3 Bigelow Laboratory for Ocean Sciences cific evolutionary relationships among these major P.O. Box 475 groups. This uncertainty is reflected in the plethora of 180 McKown Point Road published molecular phylogenies that propose virtually West Boothbay Harbor, Maine 04575 all of the possible alternative tree topologies involving 4 American Type Culture Collection Choanoflagellata, Fungi, Ichthyosporea, and Metazoa. 10801 University Boulevard For example, a monophyletic MetazoaϩChoanoflagel- Manassas, Virginia 20110 lata group has been suggested on the basis of small 5 Department of Biochemistry subunit (SSU) rDNA sequences [1, 6, 7]. Other studies and Molecular Biology using the same sequences have allied Choanoflagellata Dalhousie University with the Fungi [8], placed Choanoflagellata prior to the Halifax, Nova Scotia B3H 4H7 divergence of animals and Fungi [9], or even placed Canada them prior to the divergence of green algae and land plants [10]. -

Fungal Evolution: Major Ecological Adaptations and Evolutionary Transitions
Biol. Rev. (2019), pp. 000–000. 1 doi: 10.1111/brv.12510 Fungal evolution: major ecological adaptations and evolutionary transitions Miguel A. Naranjo-Ortiz1 and Toni Gabaldon´ 1,2,3∗ 1Department of Genomics and Bioinformatics, Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Dr. Aiguader 88, Barcelona 08003, Spain 2 Department of Experimental and Health Sciences, Universitat Pompeu Fabra (UPF), 08003 Barcelona, Spain 3ICREA, Pg. Lluís Companys 23, 08010 Barcelona, Spain ABSTRACT Fungi are a highly diverse group of heterotrophic eukaryotes characterized by the absence of phagotrophy and the presence of a chitinous cell wall. While unicellular fungi are far from rare, part of the evolutionary success of the group resides in their ability to grow indefinitely as a cylindrical multinucleated cell (hypha). Armed with these morphological traits and with an extremely high metabolical diversity, fungi have conquered numerous ecological niches and have shaped a whole world of interactions with other living organisms. Herein we survey the main evolutionary and ecological processes that have guided fungal diversity. We will first review the ecology and evolution of the zoosporic lineages and the process of terrestrialization, as one of the major evolutionary transitions in this kingdom. Several plausible scenarios have been proposed for fungal terrestralization and we here propose a new scenario, which considers icy environments as a transitory niche between water and emerged land. We then focus on exploring the main ecological relationships of Fungi with other organisms (other fungi, protozoans, animals and plants), as well as the origin of adaptations to certain specialized ecological niches within the group (lichens, black fungi and yeasts). -

Sex Is a Ubiquitous, Ancient, and Inherent Attribute of Eukaryotic Life
PAPER Sex is a ubiquitous, ancient, and inherent attribute of COLLOQUIUM eukaryotic life Dave Speijera,1, Julius Lukešb,c, and Marek Eliášd,1 aDepartment of Medical Biochemistry, Academic Medical Center, University of Amsterdam, 1105 AZ, Amsterdam, The Netherlands; bInstitute of Parasitology, Biology Centre, Czech Academy of Sciences, and Faculty of Sciences, University of South Bohemia, 370 05 Ceské Budejovice, Czech Republic; cCanadian Institute for Advanced Research, Toronto, ON, Canada M5G 1Z8; and dDepartment of Biology and Ecology, University of Ostrava, 710 00 Ostrava, Czech Republic Edited by John C. Avise, University of California, Irvine, CA, and approved April 8, 2015 (received for review February 14, 2015) Sexual reproduction and clonality in eukaryotes are mostly Sex in Eukaryotic Microorganisms: More Voyeurs Needed seen as exclusive, the latter being rather exceptional. This view Whereas absence of sex is considered as something scandalous for might be biased by focusing almost exclusively on metazoans. a zoologist, scientists studying protists, which represent the ma- We analyze and discuss reproduction in the context of extant jority of extant eukaryotic diversity (2), are much more ready to eukaryotic diversity, paying special attention to protists. We accept that a particular eukaryotic group has not shown any evi- present results of phylogenetically extended searches for ho- dence of sexual processes. Although sex is very well documented mologs of two proteins functioning in cell and nuclear fusion, in many protist groups, and members of some taxa, such as ciliates respectively (HAP2 and GEX1), providing indirect evidence for (Alveolata), diatoms (Stramenopiles), or green algae (Chlor- these processes in several eukaryotic lineages where sex has oplastida), even serve as models to study various aspects of sex- – not been observed yet. -

Multigene Eukaryote Phylogeny Reveals the Likely Protozoan Ancestors of Opis- Thokonts (Animals, Fungi, Choanozoans) and Amoebozoa
Accepted Manuscript Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opis- thokonts (animals, fungi, choanozoans) and Amoebozoa Thomas Cavalier-Smith, Ema E. Chao, Elizabeth A. Snell, Cédric Berney, Anna Maria Fiore-Donno, Rhodri Lewis PII: S1055-7903(14)00279-6 DOI: http://dx.doi.org/10.1016/j.ympev.2014.08.012 Reference: YMPEV 4996 To appear in: Molecular Phylogenetics and Evolution Received Date: 24 January 2014 Revised Date: 2 August 2014 Accepted Date: 11 August 2014 Please cite this article as: Cavalier-Smith, T., Chao, E.E., Snell, E.A., Berney, C., Fiore-Donno, A.M., Lewis, R., Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts (animals, fungi, choanozoans) and Amoebozoa, Molecular Phylogenetics and Evolution (2014), doi: http://dx.doi.org/10.1016/ j.ympev.2014.08.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 1 Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts 2 (animals, fungi, choanozoans) and Amoebozoa 3 4 Thomas Cavalier-Smith1, Ema E. Chao1, Elizabeth A. Snell1, Cédric Berney1,2, Anna Maria 5 Fiore-Donno1,3, and Rhodri Lewis1 6 7 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK. -
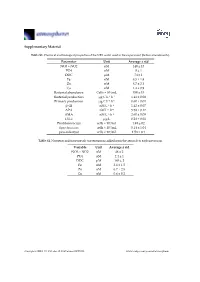
Supplementary Material Parameter Unit Average ± Std NO3 + NO2 Nm
Supplementary Material Table S1. Chemical and biological properties of the NRS water used in the experiment (before amendments). Parameter Unit Average ± std NO3 + NO2 nM 140 ± 13 PO4 nM 8 ± 1 DOC μM 74 ± 1 Fe nM 8.5 ± 1.8 Zn nM 8.7 ± 2.1 Cu nM 1.4 ± 0.9 Bacterial abundance Cells × 104/mL 350 ± 15 Bacterial production μg C L−1 h−1 1.41 ± 0.08 Primary production μg C L−1 h−1 0.60 ± 0.01 β-Gl nM L−1 h−1 1.42 ± 0.07 APA nM L−1 h−1 5.58 ± 0.17 AMA nM L−1·h−1 2.60 ± 0.09 Chl-a μg/L 0.28 ± 0.01 Prochlorococcus cells × 104/mL 1.49 ± 02 Synechococcus cells × 104/mL 5.14 ± 1.04 pico-eukaryot cells × 103/mL 1.58 × 0.1 Table S2. Nutrients and trace metals concentrations added from the aerosols to each mesocosm. Variable Unit Average ± std NO3 + NO2 nM 48 ± 2 PO4 nM 2.4 ± 1 DOC μM 165 ± 2 Fe nM 2.6 ± 1.5 Zn nM 6.7 ± 2.5 Cu nM 0.6 ± 0.2 Atmosphere 2019, 10, 358; doi:10.3390/atmos10070358 www.mdpi.com/journal/atmosphere Atmosphere 2019, 10, 358 2 of 6 Table S3. ANOVA test results between control, ‘UV-treated’ and ‘live-dust’ treatments at 20 h or 44 h, with significantly different values shown in bold. ANOVA df Sum Sq Mean Sq F Value p-value Chl-a 20 H 2, 6 0.03, 0.02 0.02, 0 4.52 0.0634 44 H 2, 6 0.02, 0 0.01, 0 23.13 0.002 Synechococcus Abundance 20 H 2, 7 8.23 × 107, 4.11 × 107 4.11 × 107, 4.51 × 107 0.91 0.4509 44 H 2, 7 5.31 × 108, 6.97 × 107 2.65 × 108, 1.16 × 107 22.84 0.0016 Prochlorococcus Abundance 20 H 2, 8 4.22 × 107, 2.11 × 107 2.11 × 107, 2.71 × 106 7.77 0.0216 44 H 2, 8 9.02 × 107, 1.47 × 107 4.51 × 107, 2.45 × 106 18.38 0.0028 Pico-eukaryote -

Predatory Flagellates – the New Recently Discovered Deep Branches of the Eukaryotic Tree and Their Evolutionary and Ecological Significance
Protistology 14 (1), 15–22 (2020) Protistology Predatory flagellates – the new recently discovered deep branches of the eukaryotic tree and their evolutionary and ecological significance Denis V. Tikhonenkov Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok, 152742, Russia | Submitted March 20, 2020 | Accepted April 6, 2020 | Summary Predatory protists are poorly studied, although they are often representing important deep-branching evolutionary lineages and new eukaryotic supergroups. This short review/opinion paper is inspired by the recent discoveries of various predatory flagellates, which form sister groups of the giant eukaryotic clusters on phylogenetic trees, and illustrate an ancestral state of one or another supergroup of eukaryotes. Here we discuss their evolutionary and ecological relevance and show that the study of such protists may be essential in addressing previously puzzling evolutionary problems, such as the origin of multicellular animals, the plastid spread trajectory, origins of photosynthesis and parasitism, evolution of mitochondrial genomes. Key words: evolution of eukaryotes, heterotrophic flagellates, mitochondrial genome, origin of animals, photosynthesis, predatory protists, tree of life Predatory flagellates and diversity of eu- of the hidden diversity of protists (Moon-van der karyotes Staay et al., 2000; López-García et al., 2001; Edg- comb et al., 2002; Massana et al., 2004; Richards The well-studied multicellular animals, plants and Bass, 2005; Tarbe et al., 2011; de Vargas et al., and fungi immediately come to mind when we hear 2015). In particular, several prevailing and very abun- the term “eukaryotes”. However, these groups of dant ribogroups such as MALV, MAST, MAOP, organisms represent a minority in the real diversity MAFO (marine alveolates, stramenopiles, opistho- of evolutionary lineages of eukaryotes. -

Protist Phylogeny and the High-Level Classification of Protozoa
Europ. J. Protistol. 39, 338–348 (2003) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Protist phylogeny and the high-level classification of Protozoa Thomas Cavalier-Smith Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK; E-mail: [email protected] Received 1 September 2003; 29 September 2003. Accepted: 29 September 2003 Protist large-scale phylogeny is briefly reviewed and a revised higher classification of the kingdom Pro- tozoa into 11 phyla presented. Complementary gene fusions reveal a fundamental bifurcation among eu- karyotes between two major clades: the ancestrally uniciliate (often unicentriolar) unikonts and the an- cestrally biciliate bikonts, which undergo ciliary transformation by converting a younger anterior cilium into a dissimilar older posterior cilium. Unikonts comprise the ancestrally unikont protozoan phylum Amoebozoa and the opisthokonts (kingdom Animalia, phylum Choanozoa, their sisters or ancestors; and kingdom Fungi). They share a derived triple-gene fusion, absent from bikonts. Bikonts contrastingly share a derived gene fusion between dihydrofolate reductase and thymidylate synthase and include plants and all other protists, comprising the protozoan infrakingdoms Rhizaria [phyla Cercozoa and Re- taria (Radiozoa, Foraminifera)] and Excavata (phyla Loukozoa, Metamonada, Euglenozoa, Percolozoa), plus the kingdom Plantae [Viridaeplantae, Rhodophyta (sisters); Glaucophyta], the chromalveolate clade, and the protozoan phylum Apusozoa (Thecomonadea, Diphylleida). Chromalveolates comprise kingdom Chromista (Cryptista, Heterokonta, Haptophyta) and the protozoan infrakingdom Alveolata [phyla Cilio- phora and Miozoa (= Protalveolata, Dinozoa, Apicomplexa)], which diverged from a common ancestor that enslaved a red alga and evolved novel plastid protein-targeting machinery via the host rough ER and the enslaved algal plasma membrane (periplastid membrane). -

Protistology Molecular Phylogeny of Aphelidium Arduennense Sp. Nov
Protistology 13 (4), 192–198 (2019) Protistology Molecular phylogeny of Aphelidium arduennense sp. nov. – new representative of Aphelida (Opis- thosporidia) Victoria S. Tcvetkova1, Natalia A. Zorina1, Maria A. Mamkaeva1 and Sergey A. Karpov1,2 1 St. Petersburg State University, St. Petersburg 199034, Russia 2 Zoological Institute, Russian Academy of Sciences, St. Petersburg 199034, Russia | Submitted November 14, 2019 | Accepted December 2, 2019 | Summary Aphelids (Aphelida) are poorly known parasitoids of algae that have raised considerable interest because of their phylogenetic position as phagotrophic protists sister to Fungi. Together with Rozellida and Microsporidia they have been classified in the Opisthosporidia but seem to be more closely related to the Fungi rather than to the Cryptomycota and Microsporidia, the other members of the Opisthosporidia. Molecular environmental studies have revealed high genetic diversity within the aphelids, but only four genera have been described: Aphelidium, Amoeboaphelidium, Paraphelidium and Pseudaphelidium. Here, we describe the life cycle of a new species of Aphelidium, Aph. arduennense. Molecular phylogenetic analysis of its 18S rRNA indicates that Aph. arduennense is sister to Aph. tribonematis, and together with Aph. melosirae they form a monophyletic cluster. Within the aphelids, this cluster is distantly related to Paraphelidium and Amoeboaphelidium. Key words: aphelids, Holomycota, Opisthosporidia, Rozellosporidia, taxonomy Introduction Rozellosporidia (Cryptomycota) formed the super- phylum Opisthosporidia, the deepest branch Aphelids are a divergent group of intracellular of the Holomycota lineage, separated from the parasitoids of green, yellow-green and diatom algae Fungi (Karpov et al., 2014a; Letcher et al., 2015; (Gromov, 2000; Karpov et al., 2014a). The four 2017; Torruella et al., 2015). Several biological known genera have different ecological preferences: peculiarities of the aphelids do not conform the Aphelidium, Amoeboaphelidium and Paraphelidium classical definition of the Fungi.