The Phagotrophic Origin of Eukaryotes and Phylogenetic Classification Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basal Body Structure and Composition in the Apicomplexans Toxoplasma and Plasmodium Maria E
Francia et al. Cilia (2016) 5:3 DOI 10.1186/s13630-016-0025-5 Cilia REVIEW Open Access Basal body structure and composition in the apicomplexans Toxoplasma and Plasmodium Maria E. Francia1* , Jean‑Francois Dubremetz2 and Naomi S. Morrissette3 Abstract The phylum Apicomplexa encompasses numerous important human and animal disease-causing parasites, includ‑ ing the Plasmodium species, and Toxoplasma gondii, causative agents of malaria and toxoplasmosis, respectively. Apicomplexans proliferate by asexual replication and can also undergo sexual recombination. Most life cycle stages of the parasite lack flagella; these structures only appear on male gametes. Although male gametes (microgametes) assemble a typical 9 2 axoneme, the structure of the templating basal body is poorly defined. Moreover, the rela‑ tionship between asexual+ stage centrioles and microgamete basal bodies remains unclear. While asexual stages of Plasmodium lack defined centriole structures, the asexual stages of Toxoplasma and closely related coccidian api‑ complexans contain centrioles that consist of nine singlet microtubules and a central tubule. There are relatively few ultra-structural images of Toxoplasma microgametes, which only develop in cat intestinal epithelium. Only a subset of these include sections through the basal body: to date, none have unambiguously captured organization of the basal body structure. Moreover, it is unclear whether this basal body is derived from pre-existing asexual stage centrioles or is synthesized de novo. Basal bodies in Plasmodium microgametes are thought to be synthesized de novo, and their assembly remains ill-defined. Apicomplexan genomes harbor genes encoding δ- and ε-tubulin homologs, potentially enabling these parasites to assemble a typical triplet basal body structure. -

Eukaryotic Microorganisms Algae and Protozoans 2
Eukaryotic Microorganisms Algae and Protozoans 2 Eukaryotic Microorganisms . prominent members of ecosystems . useful as model systems and industry . some are major human pathogens . two groups . protists . fungi 3 Kingdom Protista . Algae - eukaryotic organisms, usually unicellular and colonial, that photosynthesize with chlorophyll a . Protozoa - unicellular eukaryotes that lack tissues and share similarities in cell structure, nutrition, life cycle, and biochemistry 4 Algae .Photosynthetic organisms .Microscopic forms are unicellular, colonial, filamentous .Macroscopic forms are colonial and multicellular .Contain chloroplasts with chlorophyll and other pigments .Cell wall .May or may not have flagella 5 6 Algae .Most are free-living in fresh and marine water – plankton .Provide basis of food web in most aquatic habitats .Produce large proportion of atmospheric O2 .Dinoflagellates can cause red tides and give off toxins that cause food poisoning with neurological symptoms .Classified according to types of pigments and cell wall .Used for cosmetics, food, and medical products 7 Protozoa Protozoa 9 .Diverse group of 65,000 species .Vary in shape, lack a cell wall .Most are unicellular; colonies are rare .Most are harmless, free-living in a moist habitat .Some are animal parasites and can be spread by insect vectors .All are heterotrophic – lack chloroplasts .Cytoplasm divided into ectoplasm and endoplasm .Feed by engulfing other microbes and organic matter Protozoa 10 .Most have locomotor structures – flagella, cilia, or pseudopods .Exist as trophozoite – motile feeding stage .Many can enter into a dormant resting stage when conditions are unfavorable for growth and feeding – cyst .All reproduce asexually, mitosis or multiple fission; many also reproduce sexually – conjugation Figure 5.27 11 Protozoan Identification 12 . -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -
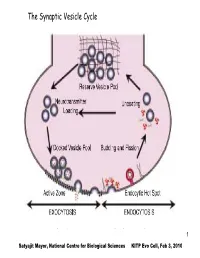
The Synaptic Vesicle Cycle
The Synaptic Vesicle Cycle 1 Satyajit Mayor, National Centre for Biological Sciences KITP Evo Cell, Feb 3, 2010 The Eukaryotic Commandments: T. Cavalier-Smith (i)origin of the endomembrane system (ER, Golgi and lysosomes) and coated- vesicle budding and fusion, including endocytosis and exocytosis; (ii) origin of the cytoskeleton, centrioles, cilia and associated molecular motors; (iii) origin of the nucleus, nuclear pore complex and trans-envelope protein and RNA transport; (iv) origin of linear chromosomes with plural replicons, centromeres and telomeres; (v) origin of novel cell-cycle controls and mitotic segregation; (vi) origin of sex (syngamy, nuclear fusion and meiosis); (vii) origin of peroxisomes; (ix) origin of mitochondria; (viii) novel patterns of rRNAprocessing using small nucleolar-ribonucleoproteins (snoRNPs); (x) origin of spliceosomal introns. 2 EM of Chemical synapse mitochondria Active Zone Figure 5.3, Bear, 2001 3 T. Cavalier-Smith International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 Fig. 1. The bacterial origins of eukaryotes as a two-stage process. I phase: The ancestors of eukaryotes, the stem neomura, are shared with archaebacteria and evolved during the neomuran revolution, in which N-linked glycoproteins replaced murein peptidoglycan and 18 other suites of characters changed radically through adaptation of an ancestral actinobacterium to thermophily, as discussed in detail by Cavalier- Smith (2002a). 4 T. Cavalier-Smith International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 Fig. 1. The bacterial origins of eukaryotes as a two-stage process. II phase: In the next phase, archaebacteria and eukaryotes diverged dramatically. Archaebacteria retained the wall and therefore their general bacterial cell and genetic organization, but became adapted to even hotter and more acidic environments by substituting prenyl ether lipids for the ancestral acyl esters and making new acid- resistant flagellar shafts (Cavalier-Smith, 2002a). -

Protist Phylogeny and the High-Level Classification of Protozoa
Europ. J. Protistol. 39, 338–348 (2003) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Protist phylogeny and the high-level classification of Protozoa Thomas Cavalier-Smith Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK; E-mail: [email protected] Received 1 September 2003; 29 September 2003. Accepted: 29 September 2003 Protist large-scale phylogeny is briefly reviewed and a revised higher classification of the kingdom Pro- tozoa into 11 phyla presented. Complementary gene fusions reveal a fundamental bifurcation among eu- karyotes between two major clades: the ancestrally uniciliate (often unicentriolar) unikonts and the an- cestrally biciliate bikonts, which undergo ciliary transformation by converting a younger anterior cilium into a dissimilar older posterior cilium. Unikonts comprise the ancestrally unikont protozoan phylum Amoebozoa and the opisthokonts (kingdom Animalia, phylum Choanozoa, their sisters or ancestors; and kingdom Fungi). They share a derived triple-gene fusion, absent from bikonts. Bikonts contrastingly share a derived gene fusion between dihydrofolate reductase and thymidylate synthase and include plants and all other protists, comprising the protozoan infrakingdoms Rhizaria [phyla Cercozoa and Re- taria (Radiozoa, Foraminifera)] and Excavata (phyla Loukozoa, Metamonada, Euglenozoa, Percolozoa), plus the kingdom Plantae [Viridaeplantae, Rhodophyta (sisters); Glaucophyta], the chromalveolate clade, and the protozoan phylum Apusozoa (Thecomonadea, Diphylleida). Chromalveolates comprise kingdom Chromista (Cryptista, Heterokonta, Haptophyta) and the protozoan infrakingdom Alveolata [phyla Cilio- phora and Miozoa (= Protalveolata, Dinozoa, Apicomplexa)], which diverged from a common ancestor that enslaved a red alga and evolved novel plastid protein-targeting machinery via the host rough ER and the enslaved algal plasma membrane (periplastid membrane). -

CH28 PROTISTS.Pptx
9/29/14 Biosc 41 Announcements 9/29 Review: History of Life v Quick review followed by lecture quiz (history & v How long ago is Earth thought to have formed? phylogeny) v What is thought to have been the first genetic material? v Lecture: Protists v Are we tetrapods? v Lab: Protozoa (animal-like protists) v Most atmospheric oxygen comes from photosynthesis v Lab exam 1 is Wed! (does not cover today’s lab) § Since many of the first organisms were photosynthetic (i.e. cyanobacteria), a LOT of excess oxygen accumulated (O2 revolution) § Some organisms adapted to use it (aerobic respiration) Review: History of Life Review: Phylogeny v Which organelles are thought to have originated as v Homology is similarity due to shared ancestry endosymbionts? v Analogy is similarity due to convergent evolution v During what event did fossils resembling modern taxa suddenly appear en masse? v A valid clade is monophyletic, meaning it consists of the ancestor taxon and all its descendants v How many mass extinctions seem to have occurred during v A paraphyletic grouping consists of an ancestral species and Earth’s history? Describe one? some, but not all, of the descendants v When is adaptive radiation likely to occur? v A polyphyletic grouping includes distantly related species but does not include their most recent common ancestor v Maximum parsimony assumes the tree requiring the fewest evolutionary events is most likely Quiz 3 (History and Phylogeny) BIOSC 041 1. How long ago is Earth thought to have formed? 2. Why might many organisms have evolved to use aerobic respiration? PROTISTS! Reference: Chapter 28 3. -

Pusillus Poseidon's Guide to Protozoa
Pusillus Poseidon’s guide to PROTOZOA GENERAL NOTES ABOUT PROTOZOANS Protozoa are also called protists. The word “protist” is the more general term and includes all types of single-celled eukaryotes, whereas “protozoa” is more often used to describe the protists that are animal-like (as opposed to plant-like or fungi-like). Protists are measured using units called microns. There are 1000 microns in one millimeter. A millimeter is the smallest unit on a metric ruler and can be estimated with your fingers: The traditional way of classifying protists is by the way they look (morphology), by the way they move (mo- tility), and how and what they eat. This gives us terms such as ciliates, flagellates, ameboids, and all those colors of algae. Recently, the classification system has been overhauled and has become immensely complicated. (Infor- mation about DNA is now the primary consideration for classification, rather than how a creature looks or acts.) If you research these creatures on Wikipedia, you will see this new system being used. Bear in mind, however, that the categories are constantly shifting as we learn more and more about protist DNA. Here is a visual overview that might help you understand the wide range of similarities and differences. Some organisms fit into more than one category and some don’t fit well into any category. Always remember that classification is an artificial construct made by humans. The organisms don’t know anything about it and they don’t care what we think! CILIATES Eats anything smaller than Blepharisma looks slightly pink because it Blepharisma itself, even smaller Bleph- makes a red pigment that senses light (simi- arismas. -

The Ciliated Protozoa Characterization, Classification, and Guide to the Literature
D. Lynn The Ciliated Protozoa Characterization, Classification, and Guide to the Literature ▶ Completely revised and updated ▶ Major chapter on The Ciliate Taxa ▶ Glossary with more than 700 terms ▶ Contains hundreds of illustrations ▶ Contains both Subject and Systematic indices The third edition of The Ciliated Protozoa continues the innovative approach of the previous two editions, thoroughly documenting the progress in our understanding of the evolutionary diversification of these widely distributed eukaryotic microorganisms. 3rd ed. 2008, XXXIII, 605 p. The Glossary is considerably revised and expanded, serving as an illustrated ‘subject index’ of more than 700 terms. An introduction to the phylum is followed by chapters on the 11 classes. Each class chapter contains 7 sections: Printed book Hardcover • taxonomic structure ▶ 279,99 € | £249.99 | $349.99 • life history and ecology ▶ *299,59 € (D) | 307,99 € (A) | CHF 330.50 • somatic structures • oral structures eBook • division and morphogenesis Available from your bookstore or • nuclei, sexuality, and life cycle other features ▶ springer.com/shop • The book includes new data on the ultrastructure of the somatic cortex of each class, MyCopy molecular phylogenetics, ecology, and on other important aspects of ciliate biology. These Printed eBook for just new data are used, along with a novel conceptual approach, to rationalize a new system ▶ € | $ 24.99 of classification for the phylum, presented in a major chapter on The CiliateTaxa. The book includes an up-to-date bibliography of approximately 3,000 citations to both the ‘classical’ ▶ springer.com/mycopy and recent literature, and both a Subject Index and a Systematic Index. This unique and timely book will serve as a comprehensive and authoritative reference work for students, teachers, and researchers who have an interest in the protozoa, and particularly the ciliates. -

Origin of the Cell Nucleus, Mitosis and Sex: Roles of Intracellular Coevolution Thomas Cavalier-Smith*
Cavalier-Smith Biology Direct 2010, 5:7 http://www.biology-direct.com/content/5/1/7 RESEARCH Open Access Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution Thomas Cavalier-Smith* Abstract Background: The transition from prokaryotes to eukaryotes was the most radical change in cell organisation since life began, with the largest ever burst of gene duplication and novelty. According to the coevolutionary theory of eukaryote origins, the fundamental innovations were the concerted origins of the endomembrane system and cytoskeleton, subsequently recruited to form the cell nucleus and coevolving mitotic apparatus, with numerous genetic eukaryotic novelties inevitable consequences of this compartmentation and novel DNA segregation mechanism. Physical and mutational mechanisms of origin of the nucleus are seldom considered beyond the long- standing assumption that it involved wrapping pre-existing endomembranes around chromatin. Discussions on the origin of sex typically overlook its association with protozoan entry into dormant walled cysts and the likely simultaneous coevolutionary, not sequential, origin of mitosis and meiosis. Results: I elucidate nuclear and mitotic coevolution, explaining the origins of dicer and small centromeric RNAs for positionally controlling centromeric heterochromatin, and how 27 major features of the cell nucleus evolved in four logical stages, making both mechanisms and selective advantages explicit: two initial stages (origin of 30 nm chromatin fibres, enabling DNA compaction; and firmer attachment of endomembranes to heterochromatin) protected DNA and nascent RNA from shearing by novel molecular motors mediating vesicle transport, division, and cytoplasmic motility. Then octagonal nuclear pore complexes (NPCs) arguably evolved from COPII coated vesicle proteins trapped in clumps by Ran GTPase-mediated cisternal fusion that generated the fenestrated nuclear envelope, preventing lethal complete cisternal fusion, and allowing passive protein and RNA exchange. -

Protists – a Textbook Example for a Paraphyletic Taxon
ARTICLE IN PRESS Organisms, Diversity & Evolution 7 (2007) 166–172 www.elsevier.de/ode Protists – A textbook example for a paraphyletic taxon$ Martin Schlegela,Ã, Norbert Hu¨lsmannb aInstitute for Biology II, University of Leipzig, Talstraße 33, 04103 Leipzig, Germany bFree University of Berlin, Institute of Biology/Zoology, Working group Protozoology, Ko¨nigin-Luise-Straße 1-3, 14195 Berlin, Germany Received 7 September 2004; accepted 21 November 2006 Abstract Protists constitute a paraphyletic taxon since the latter is based on the plesiomorphic character of unicellularity and does not contain all descendants of the stem species. Multicellularity evolved several times independently in metazoans, higher fungi, heterokonts, red and green algae. Various hypotheses have been developed on the evolution and nature of the eukaryotic cell, considering the accumulating data on the chimeric nature of the eukaryote genome. Subsequent evolution of the protists was further complicated by primary, secondary, and even tertiary intertaxonic recombinations. However, multi-gene sequence comparisons and structural data point to a managable number of such events. Several putative monophyletic lineages and a gross picture of eukaryote phylogeny are emerging on the basis of those data. The Chromalveolata comprise Chromista and Alveolata (Dinoflagellata, Apicomplexa, Ciliophora, Perkinsozoa, and Haplospora). Major lineages of the former ‘amoebae’ group within the Heterolobosa, Cercozoa, and Amoebozoa. Cercozoa, including filose testate amoebae, chlorarachnids, and plasmodiophoreans seem to be affiliated with foraminiferans. Amoebozoa consistently form the sister group of the Opisthokonta (including fungi, and with choanoflagellates as sister group of metazoans). A clade of ‘plants’ comprises glaucocystophytes, red algae, green algae, and land vascular plants. The controversial debate on the root of the eukaryote tree has been accelerated by the interpretation of gene fusions as apomorphic characters. -

"Plasmodium". In: Encyclopedia of Life Sciences (ELS)
Plasmodium Advanced article Lawrence H Bannister, King’s College London, London, UK Article Contents . Introduction and Description of Plasmodium Irwin W Sherman, University of California, Riverside, California, USA . Plasmodium Hosts Based in part on the previous version of this Encyclopedia of Life Sciences . Life Cycle (ELS) article, Plasmodium by Irwin W Sherman. Asexual Blood Stages . Intracellular Asexual Blood Parasite Stages . Sexual Stages . Mosquito Asexual Stages . Pre-erythrocytic Stages . Metabolism . The Plasmodium Genome . Motility . Recent History of Plasmodium Research . Evolution of Plasmodium . Conclusion Online posting date: 15th December 2009 Plasmodium is a genus of parasitic protozoa which infect Introduction and Description of erythrocytes of vertebrates and cause malaria. Their life cycle alternates between mosquito and vertebrate hosts. Plasmodium Parasites enter the bloodstream after a mosquito bite, Parasites of the genus Plasmodium are protozoans which and multiply sequentially within liver cells and erythro- invade and multiply within erythrocytes of vertebrates, and cytes before becoming male or female sexual forms. When are transmitted by mosquitoes. The motile invasive stages ingested by a mosquito, these fuse, then the parasite (merozoite, ookinete and sporozoite) are elongate, uni- multiplies again to form more invasive stages which are nucleate cells able to enter cells or pass through tissues, transmitted back in the insect’s saliva to a vertebrate. All using specialized secretory and locomotory organelles. -

Lab Exercise: Diversity of Eukaryotic Microbes OBJECTIVES
Lab Exercise: Diversity of Eukaryotic Microbes OBJECTIVES 1. To observe representatives of major types of microbes. 2. To cultivate select representatives of major types of microbes. 3. Understand key characteristics of the different eukaryotic microbes and groups found within these Kingdoms. INTRODUCTION Eukaryotic organisms have a nucleus in a membrane. They are typically more complex than prokaryotic organisms. They make up the Domain Eukarya and include the major kingdoms of Protista, Fungi, Plantae and Animalia. Protista is a diverse group that includes many different types of organisms, divided into the animal-like protists, or protozoa, and the plant-like protists, or algae. Protozoa are examples of Protistans that we will survey in this lab. Fungi are a kingdom of organisms that may be unicellular or multicellar, yeasts or molds. All fungi absorb their nutrients from their environment. Animals are multicelluar organisms that ingest their nutrients. This lab will be presented in three parts, one focusing on Protozoa, one on Fungi and one on Helminths (parasitic worms of the animal kingdom). Protozoa These are unicelluar eukaryotes. They do not have cell walls, but do have a membrane called a pellicle surrounding the cell. They have a nucleus and membrane bound organelles. They typically form cysts, a hardy dormant life-form that allows survival of harsh environments. Protozoa are classified into four phyla or groups based on their means of locomotion: • Flagellates (or phylum Mastigophora) • Amoebae (or phylum Sarcodina) • Sporozoans (or phylum Apicomplexa) and • Ciliates (or phylum Ciliophora). Flagellates have flagella for locomotion. Amoebae move by use of a pseudopod. Ciliates move with the aid of multiple cilia.