Opsin Gene Expression in Larval and Adult Deep-Sea Fishes Supports a Conserved Cone-To-Rod Pathway in Teleost Visual Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi
Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 (http://www.accessscience.com/) Article by: Boschung, Herbert Department of Biological Sciences, University of Alabama, Tuscaloosa, Alabama. Gardiner, Brian Linnean Society of London, Burlington House, Piccadilly, London, United Kingdom. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.680400 (http://dx.doi.org/10.1036/1097-8542.680400) Content Morphology Euteleostei Bibliography Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi The most recent group of actinopterygians (rayfin fishes), first appearing in the Upper Triassic (Fig. 1). About 26,840 species are contained within the Teleostei, accounting for more than half of all living vertebrates and over 96% of all living fishes. Teleosts comprise 517 families, of which 69 are extinct, leaving 448 extant families; of these, about 43% have no fossil record. See also: Actinopterygii (/content/actinopterygii/009100); Osteichthyes (/content/osteichthyes/478500) Fig. 1 Cladogram showing the relationships of the extant teleosts with the other extant actinopterygians. (J. S. Nelson, Fishes of the World, 4th ed., Wiley, New York, 2006) 1 of 9 10/7/2015 1:07 PM Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 Morphology Much of the evidence for teleost monophyly (evolving from a common ancestral form) and relationships comes from the caudal skeleton and concomitant acquisition of a homocercal tail (upper and lower lobes of the caudal fin are symmetrical). This type of tail primitively results from an ontogenetic fusion of centra (bodies of vertebrae) and the possession of paired bracing bones located bilaterally along the dorsal region of the caudal skeleton, derived ontogenetically from the neural arches (uroneurals) of the ural (tail) centra. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Fish-T1K (Transcriptomes of 1,000 Fishes) Project: Large-Scale Transcriptome Data for Fish Evolution Studies Ying Sun1,2*†, Yu Huang2†, Xiaofeng Li2†, Carole C
Sun et al. GigaScience (2016) 5:18 DOI 10.1186/s13742-016-0124-7 COMMENTARY Open Access Fish-T1K (Transcriptomes of 1,000 Fishes) Project: large-scale transcriptome data for fish evolution studies Ying Sun1,2*†, Yu Huang2†, Xiaofeng Li2†, Carole C. Baldwin3, Zhuocheng Zhou4, Zhixiang Yan5, Keith A. Crandall6, Yong Zhang2, Xiaomeng Zhao2, Min Wang2,7, Alex Wong8, Chao Fang2, Xinhui Zhang2, Hai Huang9, Jose V. Lopez10, Kirk Kilfoyle10, Yong Zhang1, Guillermo Ortí6*, Byrappa Venkatesh11* and Qiong Shi2,7,12* Abstract Ray-finned fishes (Actinopterygii) represent more than 50 % of extant vertebrates and are of great evolutionary, ecologic and economic significance, but they are relatively underrepresented in ‘omics studies. Increased availability of transcriptome data for these species will allow researchers to better understand changes in gene expression, and to carry out functional analyses. An international project known as the “Transcriptomes of 1,000 Fishes” (Fish-T1K) project has been established to generate RNA-seq transcriptome sequences for 1,000 diverse species of ray-finned fishes. The first phase of this project has produced transcriptomes from more than 180 ray-finned fishes, representing 142 species and covering 51 orders and 109 families. Here we provide an overview of the goals of this project and the work done so far. Keywords: Fish-T1K, Fish, Transcriptome, RNA, Database, Biodiversity Background whole genomes of only 38 fish species have been pub- Ray-finned fishes (Actinopterygii) are the most diverse lished (Additional file 1) and, although the number is and abundant group of extant vertebrates. Thus far, ap- growing (Additional file 2), searching the National Cen- proximately 32,900 fish species are recorded in FishBase ter for Biotechnology Information (NCBI)’s Sequence [1]. -

Euteleostei: Aulopiformes) and the Timing of Deep-Sea Adaptations ⇑ Matthew P
Molecular Phylogenetics and Evolution 57 (2010) 1194–1208 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Estimating divergence times of lizardfishes and their allies (Euteleostei: Aulopiformes) and the timing of deep-sea adaptations ⇑ Matthew P. Davis a, , Christopher Fielitz b a Museum of Natural Science, Louisiana State University, 119 Foster Hall, Baton Rouge, LA 70803, USA b Department of Biology, Emory & Henry College, Emory, VA 24327, USA article info abstract Article history: The divergence times of lizardfishes (Euteleostei: Aulopiformes) are estimated utilizing a Bayesian Received 18 May 2010 approach in combination with knowledge of the fossil record of teleosts and a taxonomic review of fossil Revised 1 September 2010 aulopiform taxa. These results are integrated with a study of character evolution regarding deep-sea evo- Accepted 7 September 2010 lutionary adaptations in the clade, including simultaneous hermaphroditism and tubular eyes. Diver- Available online 18 September 2010 gence time estimations recover that the stem species of the lizardfishes arose during the Early Cretaceous/Late Jurassic in a marine environment with separate sexes, and laterally directed, round eyes. Keywords: Tubular eyes have arisen independently at different times in three deep-sea pelagic predatory aulopiform Phylogenetics lineages. Simultaneous hermaphroditism evolved a single time in the stem species of the suborder Character evolution Deep-sea Alepisauroidei, the clade of deep-sea aulopiforms during the Early Cretaceous. This result indicates the Euteleostei oldest known evolutionary event of simultaneous hermaphroditism in vertebrates, with the Alepisauroidei Hermaphroditism being the largest vertebrate clade with this reproductive strategy. Divergence times Ó 2010 Elsevier Inc. -

DEEPFISHMAN Document 5 : Review of Parasites, Pathogens
DEEPFISHMAN Management And Monitoring Of Deep-sea Fisheries And Stocks Project number: 227390 Small or medium scale focused research action Topic: FP7-KBBE-2008-1-4-02 (Deepsea fisheries management) DEEPFISHMAN Document 5 Title: Review of parasites, pathogens and contaminants of deep sea fish with a focus on their role in population health and structure Due date: none Actual submission date: 10 June 2010 Start date of the project: April 1st, 2009 Duration : 36 months Organization Name of lead coordinator: Ifremer Dissemination Level: PU (Public) Date: 10 June 2010 Review of parasites, pathogens and contaminants of deep sea fish with a focus on their role in population health and structure. Matt Longshaw & Stephen Feist Cefas Weymouth Laboratory Barrack Road, The Nothe, Weymouth, Dorset DT4 8UB 1. Introduction This review provides a summary of the parasites, pathogens and contaminant related impacts on deep sea fish normally found at depths greater than about 200m There is a clear focus on worldwide commercial species but has an emphasis on records and reports from the north east Atlantic. In particular, the focus of species following discussion were as follows: deep-water squalid sharks (e.g. Centrophorus squamosus and Centroscymnus coelolepis), black scabbardfish (Aphanopus carbo) (except in ICES area IX – fielded by Portuguese), roundnose grenadier (Coryphaenoides rupestris), orange roughy (Hoplostethus atlanticus), blue ling (Molva dypterygia), torsk (Brosme brosme), greater silver smelt (Argentina silus), Greenland halibut (Reinhardtius hippoglossoides), deep-sea redfish (Sebastes mentella), alfonsino (Beryx spp.), red blackspot seabream (Pagellus bogaraveo). However, it should be noted that in some cases no disease or contaminant data exists for these species. -

Fishes-Of-The-Salish-Sea-Pp18.Pdf
NOAA Professional Paper NMFS 18 Fishes of the Salish Sea: a compilation and distributional analysis Theodore W. Pietsch James W. Orr September 2015 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce Papers NMFS National Oceanic and Atmospheric Administration Kathryn D. Sullivan Scientifi c Editor Administrator Richard Langton National Marine Fisheries Service National Marine Northeast Fisheries Science Center Fisheries Service Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Offi ce of Science and Technology Fisheries Research and Monitoring Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientifi c Publications Offi ce 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service - The NOAA Professional Paper NMFS (ISSN 1931-4590) series is published by the Scientifi c Publications Offi ce, National Marine Fisheries Service, The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original NOAA, 7600 Sand Point Way NE, research reports, taxonomic keys, species synopses, fl ora and fauna studies, and data- Seattle, WA 98115. intensive reports on investigations in fi shery science, engineering, and economics. The Secretary of Commerce has Copies of the NOAA Professional Paper NMFS series are available free in limited determined that the publication of numbers to government agencies, both federal and state. They are also available in this series is necessary in the transac- exchange for other scientifi c and technical publications in the marine sciences. -

The Exceptional Diversity of Visual Adaptations in Deep-Sea Teleost Fishes
Seminars in Cell and Developmental Biology xxx (xxxx) xxx–xxx Contents lists available at ScienceDirect Seminars in Cell & Developmental Biology journal homepage: www.elsevier.com/locate/semcdb Review The exceptional diversity of visual adaptations in deep-sea teleost fishes Fanny de Busserolles*, Lily Fogg, Fabio Cortesi, Justin Marshall Queensland Brain Institute, The University of Queensland, St Lucia, Queensland 4072, Australia ARTICLE INFO ABSTRACT Keywords: The deep-sea is the largest and one of the dimmest habitats on earth. In this extreme environment, every photon Deep-sea teleost counts and may make the difference between life and death for its inhabitants. Two sources of light are present Dim-light vision in the deep-sea; downwelling light, that becomes dimmer and spectrally narrower with increasing depth until Ocular adaptation completely disappearing at around 1000 m, and bioluminescence, the light emitted by animals themselves. Retina Despite these relatively dark and inhospitable conditions, many teleost fish have made the deep-sea their home, Opsin relying heavily on vision to survive. Their visual systems have had to adapt, sometimes in astonishing and Bioluminescence bizarre ways. This review examines some aspects of the visual system of deep-sea teleosts and highlights the exceptional diversity in both optical and retinal specialisations. We also reveal how widespread several of these adaptations are across the deep-sea teleost phylogeny. Finally, the significance of some recent findings as well as the surprising diversity in visual adaptations is discussed. 1. Introduction or mate detection, to communicate, camouflage, or for navigation, in- cluding to stay within a particular depth range [4,5]. -
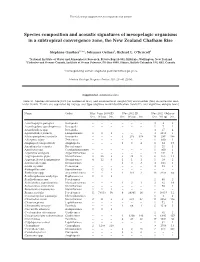
Marine Ecology Progress Series 503:23
The following supplement accompanies the article 1 Species composition and acoustic signatures of mesopelagic organisms in a subtropical convergence zone, the New Zealand Chatham Rise Stéphane Gauthier1, 2,*, Johannes Oeffner1, Richard L. O’Driscoll1 1National Institute of Water and Atmospheric Research, Private Bag 14-901, Kilbirnie, Wellington, New Zealand 2Fisheries and Oceans Canada, Institute of Ocean Sciences, PO Box 6000, Sidney, British Columbia V8L 4B2, Canada *Corresponding author: [email protected] Marine Ecology Progress Series: 503: 23–40 (2014) Supplement. Additional data Table S1. Species occurrence (Occ.) in number of tows, and abundance in weight (Wt) and number (No.) in successful mid - water trawls. Trawls are separated by voyage and type (daytime mark identification trawls ID, and nighttime oblique tows) Name Order May−June 2008 ID Nov 2011 ID Nov 2011 Oblique Occ. Wt (g) No. Occ. Wt (g) No. Occ. Wt (g) No. Acanthephyra pelagica Decapoda − − − − − − 3 9 3 Acanthephyra quadrispinosa Decapoda − − − − − − 1 7 1 Acanthephyra spp. Decapoda − − − − − − 3 17 4 Agrostichthys parkeri Lampriformes 1 8 1 − − − 3 1813 3 Alainopasiphaea australis Decapoda − − − 3 273 356 14 287 366 Allocyttus niger Zeiformes − − − − − − 1 600 1 Amphipod (unspecified) Amphipoda − − − 1 3 4 8 34 17 Anoplogaster cornuta Beryciformes − − − − − − 1 25 1 Apristurus spp. Carcharhiniformes − − − − − − 1 900 1 Argentina elongata Argentiniformes − − − − − − 4 541 6 Argyropelecus gigas Stomiiformes 2 20 2 2 38 5 8 165 12 Argyropelecus hemigymnus Stomiiformes 4 12 6 1 1 1 1 19 2 Astronesthes spp. Stomiiformes − − − 1 3 3 3 103 3 Atolla wyvillei Coronatae − − − − − − 4 83 7 Bathophilus ater Stomiiformes 1 12 1 − − − − − − Bathylagus spp. -

BMC Evolutionary Biology, 9(1)
UCLA UCLA Previously Published Works Title Did genome duplication drive the origin of teleosts? A comparative study of diversification in ray-finned fishes. Permalink https://escholarship.org/uc/item/63w7v16f Journal BMC evolutionary biology, 9(1) ISSN 1471-2148 Authors Santini, Francesco Harmon, Luke J Carnevale, Giorgio et al. Publication Date 2009-08-08 DOI 10.1186/1471-2148-9-194 Peer reviewed eScholarship.org Powered by the California Digital Library University of California BMC Evolutionary Biology BioMed Central Research article Open Access Did genome duplication drive the origin of teleosts? A comparative study of diversification in ray-finned fishes Francesco Santini1, Luke J Harmon2, Giorgio Carnevale3,4 and Michael E Alfaro*1 Address: 1Department of Ecology and Evolutionary Biology, University of California at Los Angeles, 651 Charles Young Dr. South, Los Angeles, CA 90095, USA, 2Department of Biology, University of Idaho, Moscow, ID 83844, USA, 3Dipartimento di Scienze della Terra, Universitá di Pisa, Via Santa Maria 53, 56126 Pisa, Italy and 4Museo di Storia Naturale e del Territorio, Via Roma 79, 56011, Calci, Italy Email: Francesco Santini - [email protected]; Luke J Harmon - [email protected]; Giorgio Carnevale - [email protected]; Michael E Alfaro* - [email protected] * Corresponding author Published: 8 August 2009 Received: 9 December 2008 Accepted: 8 August 2009 BMC Evolutionary Biology 2009, 9:194 doi:10.1186/1471-2148-9-194 This article is available from: http://www.biomedcentral.com/1471-2148/9/194 © 2009 Santini et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

LMR/CF/03/08 Final Report
BCLME project LMR/CF/03/08 Report on BCLME project LMR/CF/03/08 Benguela Environment Fisheries In teraction & Training Programme Review of the state of knowledge, research (past and present) of the distribution, biology, ecology, and abundance of non-exploited mesopelagic fish (Order Anguilliformes, Argentiniformes, Stomiiformes, Myctophiformes, Aulopiformes) and the bearded goby (Sufflogobius bibarbatus) in the Benguela Ecosystem. A. Staby1 and J-O. Krakstad2 1 University of Bergen, Norway 2 Institute of Marine Research, Centre for Development Cooperation, Bergen, Norway 1 BCLME project LMR/CF/03/08 Table of Contents 1 Introduction................................................................................................................. 4 2 Materials and Methods................................................................................................ 7 2.1 Regional data sources ........................................................................................ 7 2.1.1 Nan-Sis........................................................................................................ 7 2.1.2 NatMIRC data............................................................................................. 8 3 Overview and Results ................................................................................................. 9 3.1 Gobies ................................................................................................................. 9 3.1.1 Species identity and diversity .................................................................... -

Peng2009chap44.Pdf
Teleost fi shes (Teleostei) Zuogang Penga,c, Rui Diogob, and Shunping Hea,* Until recently, the classiA cation of teleosts pioneered aInstitute of Hydrobiology, The Chinese Academy of Sciences, by Greenwood et al. (5) and expanded on by Patterson Wuhan, 430072, China; bDepartment of Anthropology, The George and Rosen (6) has followed the arrangement proposed c Washington University, Washington, DC, 20052, USA; Present by Nelson (7) and today is still reP ected in A sh textbooks address: School of Biology, Georgia Institute of Technology, Atlanta, and papers. In it, species were placed in four major GA 30332, USA *To whom correspondence should be addressed ([email protected]) groups: Osteoglossomorpha, Elopomorpha, Otocephala, and Euteleostei. 7 is division was based on multiple morphological characters and molecular evidence. Abstract Based on morphological characters, Osteoglossomor- pha was considered as the most plesiomorphic living tel- Living Teleost fishes (~26,840 sp.) are grouped into 40 eosts by several works (6, 7). However, the anatomical orders, comprising the Infraclass Teleostei of the Class studies of Arratia (8–10) supported that elopomorphs, Actinopterygii. With few exceptions, morphological and not osteoglossomorphs, are the most plesiomor- and molecular phylogenetic analyses have supported phic extant teleosts. 7 is latter view was supported by four subdivisions within Teleostei: Osteoglossomorpha, the results of the most extensive morphologically based Elopomorpha, Otocephala (= Ostarioclupeomorpha), and cladistic analysis published so far on osteichthyan high- Euteleostei. Despite the progress that has been made in er-level phylogeny, which included 356 osteological and recent years for the systematics of certain teleost groups, myological characters and 80 terminal taxa, including the large-scale pattern of teleost phylogeny remains open. -

A Phylogenomic Approach to Reconstruct Interrelationships Of
Straube et al. BMC Evolutionary Biology (2018) 18:158 https://doi.org/10.1186/s12862-018-1267-1 RESEARCH ARTICLE Open Access A phylogenomic approach to reconstruct interrelationships of main clupeocephalan lineages with a critical discussion of morphological apomorphies Nicolas Straube1,2* , Chenhong Li3, Matthias Mertzen1,4, Hao Yuan3 and Timo Moritz1,4 Abstract Background: Previous molecular studies on the phylogeny and classification of clupeocephalan fishes revealed numerous new taxonomic entities. For re-analysing these taxa, we perform target gene capturing and subsequent next generation sequencing of putative ortholog exons of major clupeocephalan lineages. Sequence information for the RNA bait design was derived from publicly available genomes of bony fishes. Newly acquired sequence data comprising > 800 exon sequences was subsequently used for phylogenetic reconstructions. Results: Our results support monophyletic Otomorpha comprising Alepocephaliformes. Within Ostariophysi, Gonorynchiformes are sister to a clade comprising Cypriniformes, Characiformes, Siluriformes and Gymnotiformes, where the interrelationships of Characiformes, Siluriformes and Gymnotiformes remain enigmatic. Euteleosts comprise four major clades: Lepidogalaxiiformes, Protacanthopterygii, Stomiatii, and Galaxiiformes plus Neoteleostei. The monotypic Lepidogalaxiiformes form the sister-group to all remaining euteleosts. Protacanthopterygii, comprising Argentini-, Esoci- and Salmoniformes, is sister to Stomiatii (Osmeriformes and Stomiatiformes) and Galaxiiformes