Chem 263 April 11, 2006 Reductive Amination Amines Can Be
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cactus Chronicle
Volume 78 Issue 8 HolidayCACTUS Party CHRONICLE August 2013 Mission Statement: Plant of the Month The Los Angeles Cactus and Succulent Society (LACSS) cultivates the study and enjoyment Stenocactus Bursera, of cacti and succulent plants through educational programs and activities that promote the Commiphora hobby within a community of fellow enthusiasts and among the greater public. Refreshments Our next general meeting is Letters U-Z August 1 July New Members Pat Byrne Janice B. Lee Program Title: The Exotic Fauna and Flora of East Africa Lynn Ruger Anika Russell Lauren Stanton Presented by Steve Frieze Sonia Villarroel Editor Phyllis Frieze [email protected] Visit Us on the web http:// www.lacss.com Steve Frieze is a past president of the LACSS and has served the club in numerous other capac- ities during the past 25 years he has been a member. He is currently a partner of Desert Crea- tions, a plant store that sells unusual cacti and succulents. He has traveled to numerous loca- tions that house exotic plants, including Chile, Brazil, and Oaxaca Mexico which he just returned from. This presentation will concentrate on a trip to Tanzania he took three years ago and the wonderful succulent plants and animals that are endemic to this geographic location. You will have an opportunity to see specimen size plants such as Adenias and Commiphoras that over- whelm the senses. In addition, you will be exposed to fauna that you, in many cases, could reach out and touch if you had a touch of insanity. In one instance his tour stumbled across a fully grown lion sleeping the roadway no more than five feet from the vehicle that he was sitting in. -
2012 Formatted Lists
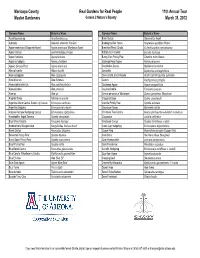
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo, -

Biodiversity As a Resource: Plant Use and Land Use Among the Shuar, Saraguros, and Mestizos in Tropical Rainforest Areas of Southern Ecuador
Biodiversity as a resource: Plant use and land use among the Shuar, Saraguros, and Mestizos in tropical rainforest areas of southern Ecuador Die Biodiversität als Ressource: Pflanzennutzung und Landnutzung der Shuar, Saraguros und Mestizos in tropischen Regenwaldgebieten Südecuadors Der Naturwissenschaftlichen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg zur Erlangung des Doktorgrades Dr. rer. nat. vorgelegt von Andrés Gerique Zipfel aus Valencia Als Dissertation genehmigt von der Naturwissenschaftlichen Fakultät der Friedrich-Alexander Universität Erlangen-Nürnberg Tag der mündlichen Prüfung: 9.12.2010 Vorsitzender der Promotionskommission: Prof. Dr. Rainer Fink Erstberichterstatterin: Prof. Dr. Perdita Pohle Zweitberichterstatter: Prof. Dr. Willibald Haffner To my father “He who seeks finds” (Matthew 7:8) ACKNOWLEDGEMENTS Firstly, I wish to express my gratitude to my supervisor, Prof. Dr. Perdita Pohle, for her trust and support. Without her guidance this study would not have been possible. I am especially indebted to Prof. Dr. Willibald Haffner as well, who recently passed away. His scientific knowledge and enthusiasm set a great example for me. I gratefully acknowledge Prof. Dr. Beck (Universität Bayreuth) and Prof. Dr. Knoke (Technische Universität München), and my colleagues and friends of the Institute of Geography (Friedrich-Alexander Universität Erlangen-Nürnberg) for sharing invaluable comments and motivation. Furthermore, I would like to express my sincere gratitude to those experts who unselfishly shared their knowledge with me, in particular to Dr. David Neill and Dr. Rainer Bussmann (Missouri Botanical Garden), Dr. Roman Krettek (Deutsche Gesellschaft für Mykologie), Dr. Jonathan Armbruster, (Auburn University, Alabama), Dr. Nathan K. Lujan (Texas A&M University), Dr. Jean Guffroy (Institut de Recherche pour le Développement, Orleans), Dr. -

Idpc Drug Policy Guide 3Rd Edition
IDPC DRUG POLICY GUIDE 3RD EDITION IDPC Drug Policy Guide 3 IDPC DRUG POLICY GUIDE 3RD EDITION Acknowledgements Global Drug Policy Observatory) • Dave Borden (StoptheDrugWar.org) IDPC would like to thank the following authors for drafting chapters of the 3rd Edition of the • Eric Gutierrez (Christian Aid) IDPC Drug Policy Guide: • Fabienne Hariga (United Nations Office on • Andrea Huber (Policy Director, Penal Reform Drugs and Crime) International) • George McBride (Beckley Foundation) • Benoit Gomis (Independent international • Gloria Lai (IDPC) security analyst, Associate Fellow at Chatham House, and Research Associate at Simon Fraser • Graham Bartlett (former Chief Superintendent University) of the Sussex Police) • Christopher Hallam (Research Officer, IDPC) • Gregor Burkhart (European Monitoring Centre for Drugs and Drug Addiction) • Coletta Youngers (Consultant, IDPC & Washington Office on Latin America) • Ines Gimenez • Diana Guzmán (Associate investigator, • Jamie Bridge (IDPC) DeJusticia, Associate Professor at Colombian • Javier Sagredo (United Nations Development National University and PhD candidate at Program) Stanford University) • Jean-Felix Savary (Groupement Romand • Diederik Lohman (Associate Director, Health d’Etudes en Addictologie) and Human Rights Division, Human Rights • Juan Fernandez Ochoa (IDPC) Watch) • Katherine Pettus (International Association for • Gloria Lai (Senior Policy Officer, IDPC) Hospice and Palliative Care) • Jamie Bridge (Senior Policy and Operations Manager, IDPC) • Luciana Pol (Centro de Estudios -

Cactus Explorers Journal
Bradleya 34/2016 pages 100–124 What is a cephalium? Root Gorelick Department of Biology and School of Mathematics & Statistics and Institute of Interdisciplinary Studies, Carleton University, 1125 Raven Road, Ottawa, Ontario K1S 5B6 Canada (e-mail: [email protected]) Photographs by the author unless otherwise stated. Summary : There are problems with previous at - gibt meist einen abgrenzbaren Übergang vom tempts to define ‘cephalium’, such as via produc - photosynthetisch aktiven Gewebe zum nicht pho - tion of more hairs and spines, confluence of tosynthetisch aktiven und blütentragenden areoles, or periderm development at or under - Cephalium, die beide vom gleichen Triebspitzen - neath each areole after flowering. I propose using meristem abstammen. Cephalien haben eine an - the term ‘cephalium’ only for a combination of dere Phyllotaxis als die vegetativen these criteria, i.e. flowering parts of cacti that Sprossabschnitte und sitzen der vorhandenen have confluent hairy or spiny areoles exterior to a vegetativen Phyllotaxis auf. Wenn blühende Ab - thick periderm, where these hairs, spines, and schnitte nur einen Teil der oben genannten Merk - periderms arise almost immediately below the male aufweisen, schlage ich vor, diese Strukturen shoot apical meristem, and with more hairs and als „Pseudocephalien“ zu bezeichnen. spines on reproductive parts than on photosyn - thetic parts of the shoot. Periderm development Introduction and confluent areoles preclude photosynthesis of Most cacti (Cactaceae) are peculiar plants, cephalia, which therefore lack or mostly lack even for angiosperms, with highly succulent stomata. There is almost always a discrete tran - stems, numerous highly lignified leaves aka sition from photosynthetic vegetative tissues to a spines, lack of functional photosynthetic leaves, non-photosynthetic flower-bearing cephalium, CAM photosynthesis, huge sunken shoot apical both of which arise from the same shoot apical meristems, and fantastic stem architectures meristem. -

The Long, Strange Trip of Mescaline
BOOKS & ARTS COMMENT harmacologists gave mescaline a fair trial. In Pthe early and mid- twentieth century, it seemed more than plausible that the fash- ionable hallucinogen could be tamed into a therapeutic agent. Mescaline: A After all, it had pro- Global History found effects on the of the First human body, and had Psychedelic been used for cen- MIKE JAY turies in parts of the Yale University Press Americas as a gateway (2019) to ceremonial spiritual experience. But this psychoactive alkaloid never found its clinical indication, as science writer Mike Jay explains in Mescaline, his anthropologi- cal and medical history. In the 1950s, the attention of biomedical researchers abruptly switched to a newly synthesized molecule with similar hallucinogenic properties but fewer physical side effects: lysergic acid di ethylamide, or LSD. First synthesized by Swiss scientist Albert Hofmann in 1938, LSD went on to become a recreational drug of choice in the 1960s hippy era. And, like mescaline, it teased psychiatrists without delivering a cure. ANCIENT HISTORY Jay traces the chronology of mescaline use. The alkaloid is found in the fast-growing San Pedro cactus (Echinopsis pachanoi) that towers above the mountainous desert scrub of the Andes, and the slow-growing, ground-hugging peyote cactus (Lophophora williamsii) native to Mexico and the south- western United States. Archaeological evidence suggests that the use of these cacti in rites of long-vanished cultures goes back at least 5,000 years. Europeans first came across peyote after Spain conquered Mexico in the early six- teenth century. (It is mentioned, for instance, in a mammoth study, The General History of the Things of New Spain, begun by scholar and friar Bernardino de Sahagún in 1529.) Attempts, largely by missionaries, to suppress WISEMAN ADAM Peyote cacti at a plant nursery in Mexico. -

Hout Vrienden Club LIGNUM
Hout Vrienden club LIGNUM Description L 007 Lignum No: 007 : Peruvian Pillar Cactus Echinopsis pachanoi (Britton &H.Friedrich & G.D. Rowley, syn. Trichocereus peruvianus Britton & Rose. Cactaceae). Local names : Cactus de San Pedro, Huachuma, Wachuma, Aguacolla Family : Cactaceae Natural distribution area : Peru Origoin of Specimen : Armenoi Apokoronou, Chania, Crete, Greece Weight by volume : approx. 400 kg/M3, tested on samples Date : September, 2018 Provided by : Willem Hurkmans Greyred discolorations in the wood Peruvian Pillar Cactus Echinopsis pachanoi in the isle of Crete General information Echinopsis comprises about 150 described species and is a large genus within the Cactyoideae, lim- ited to South America. The distinction between E. pachanoi and E. peruvianus appears to be difficult; some botanists consider them forms of the same species while others consider them to be distinct species. Date: April, 2019 Hout Vrienden club LIGNUM Habitat Echinopsis pachanoi grows at an alti- tude of 1000-3000 m asl in the Andes mountains of Peru in semi-arid areas where at night it can be cold. Flowering (mature) specimens can be from 3 to 7 metres high and can show numerous side branches. Flowers of Echinopsis pachanoi on a cutting of the same specimen that yielded the wood specimens! Since this cactus contains from 0,3% up to 1,2% of mescaline it's a psychotropic plant, the use of which can cause an altered state of consciousness. Don't try this at home. Medicinal use of E. pacanoi was already described 3,000 years ago, for example within the Chavin culture. E. pachanoi is alleged to be effective in case of high blood pressure and cardio-vascular diseases. -

February 2018
Volume 4 | Issue 1 |February 2018 Therapeutics & Toxins News Newsletter for the TDM and Toxicology Division of AACC In This Issue: Mescaline – from the hallucinogenic cactus! Mescaline, a Schedule I drug with a special exemption Peter L. Platteborze, PhD, DABCC, FAACC; Jonathan Vela* & Milo Colton PhD, JD St. Mary’s University, San Antonio, TX (*part of senior thesis project) Mescaline (3,4,5‐trimethoxyphenethylamine) is a hallucinogenic phenethylamine alkaloid found in a several plant species endemic to the Americas, most notably peyote. Human ingestion can induce a psychedelic state that is reportedly comparable to LSD and psilocybin. Native Americans have used peyote for ritualistic and medicinal purposes dating back several millennia. This long established use has led to great contention between the United States (U.S.) government and many of its indigenous people. While mescaline is listed as a Schedule I drug by the U.S. Drug Enforcement Agency, the established historical use of peyote along with sustained political lobbying successfully led to its consumption being legally permitted as an entheogen by members of the Native American Church. Plant sources: Mescaline is naturally synthesized by several members of the Cactaccea plant family to include the San Pedro cactus (Echinopsis pachanoi), the Peruvian torch cactus (E. peruviana) and most notably, peyote (Lophophora williamsii). The Echinopsis grow naturally in the Andes Mountains of South America. Mescaline is also found in very small amounts in certain members of the Fabaccea (bean) family. Of these plants, peyote contains the greatest mescaline content. Peyote is a small spineless cactus that grows in arid regions of Northern Mexico & Southern Texas (Figure 1). -

PHYTOCHEMICAL STUDY of Echinopsis Peruviana ESTUDIO
202 Recibido el 26-08-2014 Aprobado el 02-09-2014 PHYTOCHEMICAL STUDY OF Echinopsis peruviana 1 1 * Pedro A. Baldera-Aguayo y Víctor M. Reyna Pinedo ABSTRACT The “San Pedro Macho” cacti (Echinopsis peruviana or also known as Trichocereus peruviana) owes its name and importance, in part, to its taxonomical and alkaloid composition similarity to the well-known “San Pedro Hembra” cacti (Echinopsis pachanoi) or commonly referred as “San Pedro”, which is the most important species among the plants used in Traditional Medicine (TM) in Northern Perú. This phytochemical report of an Echinopsis peruviana species cultivated in Perú involves the qualitative analysis of its secondary metabolites, the quantification of the total alkaloid content and the isolation of the alkaloid mescaline, as its sulphate dihydrate salt, of the chlorenchyma in the green outer cortex of the stems of E. peruviana. ESI-MS; 1H, 13C NMR; IR; UV; elemental analysis and TLC techniques were used to characterize this compound. Key words: Echinopsis peruviana, San Pedro Macho, mescaline, phytochemical analysis, alkaloid content and phenylethylamine alkaloids. ESTUDIO FITOQUÍMICO DE Echinopsis peruviana RESUMEN El cactus “San Pedro Macho” (Echinopsis peruviana o también conocido como Trichocereus peruviana) debe en parte su nombre y su importancia a su similaridad, tanto taxonómica como en su contenido de alcaloides, con el muy conocido cactus “San Pedro Hembra” (Echinopsis pachanoi) o comúnmente denominado “San Pedro”, el cual es la especie más importante entre las plantas empleadas en Medicina Tradicional (MT) en el norte del Perú. Este trabajo fitoquímico de la especie Echinopsis peruviana cultivada en Perú comprende el análisis cualitativo de sus metabolitos secundarios, la cuantificación total de alcaloides y el aislamiento del alcaloide mescalina como su sal, el sulfato dihidratado, a partir de la corteza de los tallos del cactus E. -

Rights of Indigenous Groups
4.3 Rights of indigenous groups Introduction Key recommendations The 1989 International Labor Organization’s Con- vention concerning Indigenous and Tribal Peoples • Governments should repair the discrepan- in Independent Countries49 defines indigenous peo- cies between the UN drug conventions and ple as those who, ‘on account of their descent from international human rights agreements, to the populations which inhabited the country at the ensure that the rights of indigenous peo- time of conquest, colonisation, or the establishment ples are upheld and fully protected of present state boundaries and who, irrespective of their legal status, retain some, or all, of their own • Indigenous communities should be mean- social, economic, cultural and political institutions’, ingfully involved in the design and imple- or ‘tribal peoples in independent countries whose mentation of any policies and regulations social, cultural and economic conditions distinguish that affect them them from other sections of the national communi- • Governments should set up data collection ty, and whose status is regulated wholly or partially mechanisms to review the impact of drug by their own customs or traditions or by special laws policies and in particular drug law enforce- or regulations’. ment strategies on indigenous groups, In practical terms, this means that in addition to the and review any harmful drug law, policy or universal human rights recognised in international practice conventions (see Policy principle 2), indigenous peo- • The historical, cultural and traditional ple enjoy specific rights that protect their identity, character and potential benefits of plants culture, traditions, habitat, language and access to controlled at the national and international ancestral lands. -

Ethnobotanical Identification of Echinopsis Pachanoi in Himachal Pradesh
Ethnobotanical Identification of Echinopsis Pachanoi in Himachal Pradesh. A New Candidate for the Soma Plant? Abstract Ethnobotanical fieldwork identifies for the first time in literature the existence of the cactus Echinopsis Pachanoi in Himachal Pradesh, India. Given similarties to references to the soma plant found in vedic scriptures, the hypothesis is formulated that the plant could be a candidate for the Soma. Introduction Fieldwork carried out in Himachal Pradesh in 2013 [Di Maio] identifies a cactus plant producing a large white flower, which shares morphological traits and traditional usage with Echinopsis Pachanoi, a plant not identified by local botanical surveys carried out in the region and not recorded in scientific literature to grow in Asia. This paper reports preliminary findings and forumlates an hypotesis that if true, could mean that the identification of Echinopsis Pachanoi in this locality, or a very closely related plant would be documented for the first time. Given that Echinopsis Pachanoi is well known in the Andes for being used in divination and other shamanic rituals, and if the findings are confirmed, could this be the plant from which the Soma drink referenced in the vedic literature is extracted? Motivation Kamand Valley, Mandi District, Himachal Pradesh - India A survey was carried out om the Kamand, in Mandi District to assess the environmetal impact of a new IIT Mandi campus [Sawhey]. Among other species, the survey identified a plant with potential properties of value, Euphorbia Royleana. In comparing the survey results with notes from related fieldwork, it was noted that Euphorbia Royeleana produces small yellow flower, however visual inspections of the field had revealed sporadic presence of large white flowers on top of some of the cacti looking plants. -

Taxonomía Y Filogenia De Trichocereus (Berg.) Riccob
Naturalis Repositorio Institucional Universidad Nacional de La Plata http://naturalis.fcnym.unlp.edu.ar Facultad de Ciencias Naturales y Museo Taxonomía y filogenia de Trichocereus (Berg.) Riccob. (Trichocereeae - Cactaceae) Albesiano Hoyos, Adriana Sofía Doctor en Ciencias Naturales Dirección: Kiesling, Roberto Co-dirección: Freire, Susana Facultad de Ciencias Naturales y Museo 2015 Acceso en: http://naturalis.fcnym.unlp.edu.ar/id/20160225001427 Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional Powered by TCPDF (www.tcpdf.org) UNIVERSIDAD NACIONAL DE LA PLATA FACULTAD DE CIENCIAS NATURALES Y MUSEO ―Taxonomía y filogenia de Trichocereus (Berg.) Riccob. (Trichocereeae- Cactaceae)‖ Tesis presentada para optar al grado de Doctor en Ciencias Naturales de la Universidad Nacional de La Plata Bióloga M. Sc. Adriana Sofía Albesiano Hoyos Director: Dr. Roberto Kiesling Co-Directora: Dra. Susana Freire La Plata, Argentina Agosto 3 de 2015 COMISIÓN ASESORA Dr. Roberto Kiesling, Instituto Argentino de Investigaciones de las Zonas Áridas-IADIZA, CONICET. Dra. Susana Freire, Instituto de Botánica Darwinion-San Isidro. Prof. Titular, Cátedra de Botánica Sistemática II, Facultad Ciencias Naturales y Museo, Universidad Nacional de La Plata. DEFENSA ORAL Y PÚBLICA Lugar y Fecha: Calificación: TRIBUNAL Firma:………………………………………………………...Aclaración………… Firma:………………………………………………………...Aclaración………… Firma:………………………………………………………...Aclaración………… TABLA DE CONTENIDO AGRADECIMIENTOS .............................................................................................................................