Cactus Explorers Journal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Haseltonia Articles and Authors.Xlsx
ABCDEFG 1 CSSA "HASELTONIA" ARTICLE TITLES #1 1993–#26 2019 AUTHOR(S) R ISSUE(S) PAGES KEY WORD 1 KEY WORD 2 2 A Cactus Database for the State of Baja California, Mexico Resendiz Ruiz, María Elena 2000 7 97-99 BajaCalifornia Database A First Record of Yucca aloifolia L. (Agavaceae/Asparagaceae) Naturalized Smith, Gideon F, Figueiredo, 3 in South Africa with Notes on its uses and Reproductive Biology Estrela & Crouch, Neil R 2012 17 87-93 Yucca Fotinos, Tonya D, Clase, Teodoro, Veloz, Alberto, Jimenez, Francisco, Griffith, M A Minimally Invasive, Automated Procedure for DNA Extraction from Patrick & Wettberg, Eric JB 4 Epidermal Peels of Succulent Cacti (Cactaceae) von 2016 22 46-47 Cacti DNA 5 A Morphological Phylogeny of the Genus Conophytum N.E.Br. (Aizoaceae) Opel, Matthew R 2005 11 53-77 Conophytum 6 A New Account of Echidnopsis Hook. F. (Asclepiadaceae: Stapeliae) Plowes, Darrel CH 1993 1 65-85 Echidnopsis 7 A New Cholla (Cactaceae) from Baja California, Mexico Rebman, Jon P 1998 6 17-21 Cylindropuntia 8 A New Combination in the genus Agave Etter, Julia & Kristen, Martin 2006 12 70 Agave A New Series of the Genus Opuntia Mill. (Opuntieae, Opuntioideae, Oakley, Luis & Kiesling, 9 Cactaceae) from Austral South America Roberto 2016 22 22-30 Opuntia McCoy, Tom & Newton, 10 A New Shrubby Species of Aloe in the Imatong Mountains, Southern Sudan Leonard E 2014 19 64-65 Aloe 11 A New Species of Aloe on the Ethiopia-Sudan Border Newton, Leonard E 2002 9 14-16 Aloe A new species of Ceropegia sect. -

Cactus Chronicle
Volume 78 Issue 8 HolidayCACTUS Party CHRONICLE August 2013 Mission Statement: Plant of the Month The Los Angeles Cactus and Succulent Society (LACSS) cultivates the study and enjoyment Stenocactus Bursera, of cacti and succulent plants through educational programs and activities that promote the Commiphora hobby within a community of fellow enthusiasts and among the greater public. Refreshments Our next general meeting is Letters U-Z August 1 July New Members Pat Byrne Janice B. Lee Program Title: The Exotic Fauna and Flora of East Africa Lynn Ruger Anika Russell Lauren Stanton Presented by Steve Frieze Sonia Villarroel Editor Phyllis Frieze [email protected] Visit Us on the web http:// www.lacss.com Steve Frieze is a past president of the LACSS and has served the club in numerous other capac- ities during the past 25 years he has been a member. He is currently a partner of Desert Crea- tions, a plant store that sells unusual cacti and succulents. He has traveled to numerous loca- tions that house exotic plants, including Chile, Brazil, and Oaxaca Mexico which he just returned from. This presentation will concentrate on a trip to Tanzania he took three years ago and the wonderful succulent plants and animals that are endemic to this geographic location. You will have an opportunity to see specimen size plants such as Adenias and Commiphoras that over- whelm the senses. In addition, you will be exposed to fauna that you, in many cases, could reach out and touch if you had a touch of insanity. In one instance his tour stumbled across a fully grown lion sleeping the roadway no more than five feet from the vehicle that he was sitting in. -

Caryophyllales 2018 Instituto De Biología, UNAM September 17-23
Caryophyllales 2018 Instituto de Biología, UNAM September 17-23 LOCAL ORGANIZERS Hilda Flores-Olvera, Salvador Arias and Helga Ochoterena, IBUNAM ORGANIZING COMMITTEE Walter G. Berendsohn and Sabine von Mering, BGBM, Berlin, Germany Patricia Hernández-Ledesma, INECOL-Unidad Pátzcuaro, México Gilberto Ocampo, Universidad Autónoma de Aguascalientes, México Ivonne Sánchez del Pino, CICY, Centro de Investigación Científica de Yucatán, Mérida, Yucatán, México SCIENTIFIC COMMITTEE Thomas Borsch, BGBM, Germany Fernando O. Zuloaga, Instituto de Botánica Darwinion, Argentina Victor Sánchez Cordero, IBUNAM, México Cornelia Klak, Bolus Herbarium, Department of Biological Sciences, University of Cape Town, South Africa Hossein Akhani, Department of Plant Sciences, School of Biology, College of Science, University of Tehran, Iran Alexander P. Sukhorukov, Moscow State University, Russia Michael J. Moore, Oberlin College, USA Compilation: Helga Ochoterena / Graphic Design: Julio C. Montero, Diana Martínez GENERAL PROGRAM . 4 MONDAY Monday’s Program . 7 Monday’s Abstracts . 9 TUESDAY Tuesday ‘s Program . 16 Tuesday’s Abstracts . 19 WEDNESDAY Wednesday’s Program . 32 Wednesday’s Abstracs . 35 POSTERS Posters’ Abstracts . 47 WORKSHOPS Workshop 1 . 61 Workshop 2 . 62 PARTICIPANTS . 63 GENERAL INFORMATION . 66 4 Caryophyllales 2018 Caryophyllales General program Monday 17 Tuesday 18 Wednesday 19 Thursday 20 Friday 21 Saturday 22 Sunday 23 Workshop 1 Workshop 2 9:00-10:00 Key note talks Walter G. Michael J. Moore, Berendsohn, Sabine Ya Yang, Diego F. Registration -

Floristic and Ecological Characterization of Habitat Types on an Inselberg in Minas Gerais, Southeastern Brazil
Acta Botanica Brasilica - 31(2): 199-211. April-June 2017. doi: 10.1590/0102-33062016abb0409 Floristic and ecological characterization of habitat types on an inselberg in Minas Gerais, southeastern Brazil Luiza F. A. de Paula1*, Nara F. O. Mota2, Pedro L. Viana2 and João R. Stehmann3 Received: November 21, 2016 Accepted: March 2, 2017 . ABSTRACT Inselbergs are granitic or gneissic rock outcrops, distributed mainly in tropical and subtropical regions. Th ey are considered terrestrial islands because of their strong spatial and ecological isolation, thus harboring a set of distinct plant communities that diff er from the surrounding matrix. In Brazil, inselbergs scattered in the Atlantic Forest contain unusually high levels of plant species richness and endemism. Th is study aimed to inventory species of vascular plants and to describe the main habitat types found on an inselberg located in the state of Minas Gerais, in southeastern Brazil. A total of 89 species of vascular plants were recorded (belonging to 37 families), of which six were new to science. Th e richest family was Bromeliaceae (10 spp.), followed by Cyperaceae (seven spp.), Orchidaceae and Poaceae (six spp. each). Life forms were distributed in diff erent proportions between habitats, which suggested distinct microenvironments on the inselberg. In general, habitats under similar environmental stress shared common species and life-form proportions. We argue that fl oristic inventories are still necessary for the development of conservation strategies and management of the unique vegetation on inselbergs in Brazil. Keywords: endemism, granitic and gneissic rock outcrops, life forms, terrestrial islands, vascular plants occurring on rock outcrops within the Atlantic Forest Introduction domain, 416 are endemic to these formations (Stehmann et al. -

Cephalocereus Senilis (Old Man Cactus, Old Man of Mexico, Bunny Cactus) Old Man Cactus Is a Slender, Columnar Succulent Native to the Rocky Regions of Central Mexico
Cephalocereus senilis (Old Man Cactus, old man of Mexico, bunny cactus) Old man cactus is a slender, columnar succulent native to the rocky regions of central Mexico. It is densely covered with long, bristly, grayish white hairs that resemble those on the head of an elderly gentleman. These are pollinated by moths and bats and followed by pinkish red fruits. Landscape Information French Name: Tête de vieillard ﺻﺒﺎﺭ ﺍﻟﺮﺟﻞ ﺍﻟﻌﺠﻮﺯ :Arabic Name Pronounciation: sef-uh-low-KER-ee-us SEE- nil-is Plant Type: Cactus / Succulent Origin: Mexico, South America (Hidalgo, Mexico) Heat Zones: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Hardiness Zones: 9, 10, 11, 12, 13 Uses: Indoor, Container, Rock Garden Size/Shape Growth Rate: Slow Tree Shape: Columnar, Upright Canopy Symmetry: Symmetrical Canopy Density: Medium Canopy Texture: Coarse Height at Maturity: 8 to 15 m Spread at Maturity: 1.5 to 3 meters Time to Ultimate Height: 20 to 50 Years Plant Image Cephalocereus senilis (Old Man Cactus, old man of Mexico, bunny cactus) Botanical Description Foliage Leaf Arrangement: Whorled Leaf Persistance: Evergreen Leaf Type: Simple Leaf Blade: 5 - 10 cm Leaf Shape: Linear Leaf Textures: Hairy Leaf Scent: Pleasant Color(growing season): Silver Flower Flower Showiness: False Flower Size Range: 3 - 7 Flower Image Flower Scent: Pleasant Flower Color: Pink Seasons: Summer Trunk Number of Trunks: Single Trunk Trunk Esthetic Values: Rough Texture, Spines Fruit Fruit Showiness: False Fruit Size Range: 1.5 - 3 Fruit Colors: Green, Red, Pink Seasons: Summer -

Cactus Seed List
if J Historic, archived document Do not assume content reflects current scientific knowledge, policies, or practices. 1 AUNT PAT K E C K I V b, O mote s, mm m re EDINBURG, TEX AS 111. S. Department of Agricultu CACTUS SEED LIST Please list several substitutes. ACANTHOCALYCIUM VIOLACEUM LOBVIA ANDALGALEWSIS ACANTHOCEREUS PENTAGONUS LOBVIA BRUCHII AGAVE PARVIFLORA LOBVIA FORMOSA AGAVE VICTORIA REGINAE LOBVIA HUASCHA LOBVIA HYBRID (FORMOSA X BRO ALOE STRIATA LOBVIA LONGISPINA ASTROPHYTUM MYRIOSTIGMA LOVIA PENTLANDII ASTROPHYTUM NUDA LOBVIA HYB. ANDAL-X BRUCHII) ASTROPHYTUM ORNATUM LOBVIA SP. X BLOSSFELD (ORANGF ASTROPHYTUM HYBRID LOBVIA MIXED CARNEGIA GIGANTEA MALACOCARPUS CORYNODES CEPHALOCEREUS POLYLOPHUS MALACOCARPUS ERINACEUS CEPHALOCEREUS SENILIS MALACOCARPUS SELLOWII CEREUS ALACRIPORTANUS MALACOCARPUS VORWERKIANUS CEREUS PERUVIANUS MAMMILLARIA ALBICANS CEREUS PERUVIANUS MONS. MAM. ANGULARIS CEREUS STENAGONUS MAMMILLARIA BRAUNEANA CEREUS NO. 6 (NEW) MAMMILLARIA CELSIANA CEREUS NO. 8 MAM. COMPRESSA CEREUS NO. 17 (NEW) MAMMILLARIA FORMOSA CEREUS NO. 20 (NEW) MAM. HIDALGENSIS CLEISTOCACTUS MORAWETZIANUS MAMMILLARIA MACRACANTHA CLEISTOCACTUS' STRAUSII MAMMILLARIA ORCUTTII CLEISTOCACTUS. TUPIZENSIS MAM. PERBELLA CLEISTOCACTUS JUJUYENSIS MAM. RHODANTHA CORYPHANTHA MIXED MAM. SPINOSISSIMA CRASSULA FALCATA MAM. TETRACANTHA DYCKIA RARIFLORA MAM. VAUPELII DYCKIA SULPHUREA MAMMILLARIA MIXED ECHINOCEREUS CHIHUAHUA MELOCACTUS BAHIENSIS ECHINOCEREUS ENGELMANII MELOCACTUS ERNESTII ECHINOCEREUS M.H. 15 (PINK FL> NOTOCACTUS APRICUS ECHINOCACTUS GRUSONII -

Pilosocereus Robinii) Using New Genetic Tools Tonya D
Volume 31: Number 2 > 2014 The Quarterly Journal of the Florida Native Plant Society Palmetto Fern Conservation in a Biodiversity Hotspot ● Saving the Endangered Florida Key Tree Cactus Saving the Endangered Florida Key Tree Cactus (Pilosocereus robinii) Using New Genetic Tools Tonya D. Fotinos, Dr. Joyce Maschinski & Dr. Eric von Wettberg Biological systems around the globe are being threatened by human-induced landscape changes, habitat degradation and climate change (Barnosky et al. 2011; Lindenmayer & Fischer 2006; Thomas et al. 2004; Tilman et al. 1994). Although there is a considerable threat across the globe, numerically the threat is highest in the biodiversity hotspots of the world. Of those hotspots, South Florida and the Caribbean are considered in the top five areas for conservation action because of the high level of endemism and threat (Myers et al. 2000). South Florida contains roughly 125 endemic species and is the northernmost limit of the distribution of many tropical species (Abrahamson 1984; Gann et al. 2002). The threat to these species comes predominantly from sea level rise, which could be >1 m by the end of the century (Maschinski et al. 2011). Above: Pilosocereus robinii stand in the Florida Keys. Photo: Jennifer Possley, Center for Tropical Conservation/Fairchild Tropical Botanic Garden. Above: Pilosocereus robinii in bloom at the Center for Tropical Conservation. Photo: Devon Powell, Center for Tropical Conservation/Fairchild Tropical Botanic Garden. 12 ● The Palmetto Volume 31:2 ● 2014 Restoration of imperiled populations is a priority for mitigating the looming species extinctions (Barnosky et al. 2011). Populating new or previously occupied areas or supple- menting a local population of existing individuals are strategies that improve the odds that a population or species will survive. -

Elaboración De Una Guía Ilustrada De Cactáceas En Honduras
Elaboración de una guía ilustrada de Cactáceas en Honduras Juan Pablo Schulze Rojas ZAMORANO Carrera de Desarrollo Socioeconómico y Ambiente Diciembre, 2004 i Elaboración de una guía ilustrada de Cactáceas en Honduras Proyecto especial presentado como requisito parcial para optar al título de Ingeniero en Desarrollo Socioeconómico y Ambiente en el Grado Académico de Licenciatura. Presentado por: Juan Pablo Schulze Rojas Honduras Diciembre, 2004 ii El autor concede a Zamorano permiso para reproducir y distribuir copias de este trabajo para fines educativos. Para otras personas físicas o jurídicas se reservan los derechos de autor. ________________________________ Juan Pablo Schulze Rojas Honduras Diciembre, 2004 iii Elaboración de una guía ilustrada de Cactáceas en Honduras Presentado por Juan Pablo Schulze Rojas Aprobada: __________________________ __________________________ José L. Linares, Ing. Agr. Mayra Falck, M.Sc. Asesor Principal Coordinadora de la Carrera de Desarrollo Socioeconómico y Ambiente __________________________ __________________________ George Pilz, Ph.D. Aurelio Revilla, M.S.A. Asesor Decano Académico Interino __________________________ Kenneth L. Hoadley, D.B.A. Rector iv DEDICATORIA A mi mamá Toya. A mi papá Juanca. A mi hermano Javier. A Claire. A mis abuelitos. A mis compañeros. A todos los que me apoyaron. A la naturaleza. A la esperanza por la PAZ. v AGRADECIMIENTOS A José L. Linares, por su asesoría, alegría y buena cocina. Al Dr. Pilz, por la tranquilidad. A mis padres, por todo su gran apoyo, soporte, aguante y cariño brindado. A Javier por ser mi hermano. A los clanes Rojas y Muñoz-Reyes, por haberme acogido. A los Babos, por ser un ejemplo de valores. A la Mimi, por su alegría. -

K a K T E E N K U N D E 1 9
KAKTEENKUNDE 1 9 4 1 Veröffentlicht von der Deutschen Kakteen-Gesellschaft Inhaltsverzeichnis I. Autoren und Artikel Seite Backeberg, Curt: Wertvolle Echinocereen aus Oklahoma . 1 ff . — Seltene Cereen des westandinen Südamerikas . 16 ff ., 25 ff . — Stachlige Wildnis, 80 000 km durch die Urwelt Amerikas . 49 ff . Eberle, Dr . Wilhelm: Kakteenfreuden, 800 m ü . Meer . 44 ff . Fleischer, Zd .: Über eine Epidermiskrankheit der Kakteen . 39 ff . Iringer, Hedy: Eine hübsche Laune der Natur . 24 Krainz, H .: Die Samensammlung der Zentralforschungsstelle (ZfSt) und ihre Aufgaben . 58 ff . Krug, Werner: Furcht vor dem Winter? . 21 ff . — Etwas aus der Sämlingszucht! . 41 ff . Oesterreich, Gerhard: Einrichtung einer Kartenstelle im Rahmen der Zentralforschungs- stelleder D .K G. 47 Pütter, Karl, Ed .: Was das Ausland im zweiten Halbjahr 1939 über Kakteen berichtete . 12 ff . — Was das Ausland im ersten Halbjahr 1940 über Kakteen berichtete . 37 ff . — Was das Ausland im zweiten Halbjahr 1940 über Kakteen berichtete . 66 ff . — Zehn Jahre Arbeit für die D .K G. 70 Ryffel, A .: Einiges zur Kultur und Überwinterung von Asclepiadaceen im Winter . 72 Sadovsky, Ot .: Zucht und Pflege der Ferokakteen . 29 ff . Vergen, H .: Gedanken eines Naturfreundes . 68 ff . Viereck, H . W .: Reiseerinnerungen aus den Kakteengebieten Sonoras (Mexiko) . 7 ff . Winkelmann, Fritz: Frailea, Eine Lanze für unsere Kleinsten . 34 ff . II. Gattungen und Arten* ) (+ = Bild) Seite Seite Browningia candelaris . 26 f . + Gymnanthocerei . 25 ff . Gymnanthocereus . 25 ff . Cereus alamosensis . 11 — chlorocarpus . 26 — Bridgesii . 19 — microspermus . 25 f . + — eburneus . 39 Gymnocalycium . 15, 66 — giganteus . 9 + — immomoratum . 37 — macrogonus . 19 f . + + — pecten-aboriginum . 8, 10 f . Hylocereus polyrhizus . 52 + — peruvianus . 12 f . — venezuelensis . -

Roadrunner News Newsletter of the Long Beach Cactus Club Founded 1933; Affiliate of the Cactus and Succulent Society of America, Inc
May 2018 Roadrunner News Newsletter of the Long Beach Cactus Club Founded 1933; Affiliate of the Cactus and Succulent Society of America, Inc. Drosanthemum speciosum, photo by Krystoff Przykucki MEETING PROGRAM: Tom Glavich: “The Genus Euphorbia” LOCATION: Rancho Los Alamitos, 6400 Bixby Hill Road, Long Beach, CA 90815. We will meet in the meeting room next to the gift shop. Rancho Los Alamitos is located within Bixby Hill and accessed through the residential security gate at Anaheim and Palo Verde. From the 405 Freeway, exit at Palo Verde Avenue and turn south. From the 605 Freeway, exit at Willow, follow to Palo Verde and turn south. TIME: Sunday, May 6th, 2018 at 1:30 p.m. Setup will be from 12:30 – 1:30. Members will be working in the garden starting at 11 AM. Bring a lunch if you need to. REFRESHMENTS: We will follow the alphabet to determine who is to bring the snacks and finger foods. This month, those with last names starting with the letters A through F are asked to bring the goodies. Please feel free to bring something even if you don’t fall into this group. PLANT-OF-THE-MONTH: Cactus: Echinocactus, Ferocactus, Succulent: Monadenium, Jatropha Descriptions by Scott Bunnell: Echinocactus is a genus of cacti in the subfamily Cactoideae. It and Ferocactus are the two genera of barrel cactus. Members of the genus usually have heavy spination and relatively small flowers. The fruits are copiously woolly, which is one major distinction between Echinocactus and Ferocactus. Propagation is by seed. Perhaps the best known species is the golden barrel (Echinocactus grusonii) from Mexico, an easy-to-grow and widely cultivated plant. -

University of Florida Thesis Or Dissertation Formatting
SYSTEMATICS OF TRIBE TRICHOCEREEAE AND POPULATION GENETICS OF Haageocereus (CACTACEAE) By MÓNICA ARAKAKI MAKISHI A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2008 1 © 2008 Mónica Arakaki Makishi 2 To my parents, Bunzo and Cristina, and to my sisters and brother. 3 ACKNOWLEDGMENTS I want to express my deepest appreciation to my advisors, Douglas Soltis and Pamela Soltis, for their consistent support, encouragement and generosity of time. I would also like to thank Norris Williams and Michael Miyamoto, members of my committee, for their guidance, good disposition and positive feedback. Special thanks go to Carlos Ostolaza and Fátima Cáceres, for sharing their knowledge on Peruvian Cactaceae, and for providing essential plant material, confirmation of identifications, and their detailed observations of cacti in the field. I am indebted to the many individuals that have directly or indirectly supported me during the fieldwork: Carlos Ostolaza, Fátima Cáceres, Asunción Cano, Blanca León, José Roque, María La Torre, Richard Aguilar, Nestor Cieza, Olivier Klopfenstein, Martha Vargas, Natalia Calderón, Freddy Peláez, Yammil Ramírez, Eric Rodríguez, Percy Sandoval, and Kenneth Young (Peru); Stephan Beck, Noemí Quispe, Lorena Rey, Rosa Meneses, Alejandro Apaza, Esther Valenzuela, Mónica Zeballos, Freddy Centeno, Alfredo Fuentes, and Ramiro Lopez (Bolivia); María E. Ramírez, Mélica Muñoz, and Raquel Pinto (Chile). I thank the curators and staff of the herbaria B, F, FLAS, LPB, MO, USM, U, TEX, UNSA and ZSS, who kindly loaned specimens or made information available through electronic means. Thanks to Carlos Ostolaza for providing seeds of Haageocereus tenuis, to Graham Charles for seeds of Blossfeldia sucrensis and Acanthocalycium spiniflorum, to Donald Henne for specimens of Haageocereus lanugispinus; and to Bernard Hauser and Kent Vliet for aid with microscopy. -
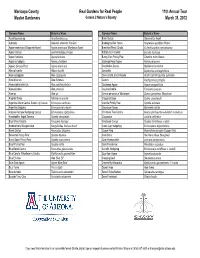
2012 Formatted Lists
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo,