PHYTOCHEMICAL STUDY of Echinopsis Peruviana ESTUDIO
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cactus Chronicle
Volume 78 Issue 8 HolidayCACTUS Party CHRONICLE August 2013 Mission Statement: Plant of the Month The Los Angeles Cactus and Succulent Society (LACSS) cultivates the study and enjoyment Stenocactus Bursera, of cacti and succulent plants through educational programs and activities that promote the Commiphora hobby within a community of fellow enthusiasts and among the greater public. Refreshments Our next general meeting is Letters U-Z August 1 July New Members Pat Byrne Janice B. Lee Program Title: The Exotic Fauna and Flora of East Africa Lynn Ruger Anika Russell Lauren Stanton Presented by Steve Frieze Sonia Villarroel Editor Phyllis Frieze [email protected] Visit Us on the web http:// www.lacss.com Steve Frieze is a past president of the LACSS and has served the club in numerous other capac- ities during the past 25 years he has been a member. He is currently a partner of Desert Crea- tions, a plant store that sells unusual cacti and succulents. He has traveled to numerous loca- tions that house exotic plants, including Chile, Brazil, and Oaxaca Mexico which he just returned from. This presentation will concentrate on a trip to Tanzania he took three years ago and the wonderful succulent plants and animals that are endemic to this geographic location. You will have an opportunity to see specimen size plants such as Adenias and Commiphoras that over- whelm the senses. In addition, you will be exposed to fauna that you, in many cases, could reach out and touch if you had a touch of insanity. In one instance his tour stumbled across a fully grown lion sleeping the roadway no more than five feet from the vehicle that he was sitting in. -

University of Florida Thesis Or Dissertation Formatting
SYSTEMATICS OF TRIBE TRICHOCEREEAE AND POPULATION GENETICS OF Haageocereus (CACTACEAE) By MÓNICA ARAKAKI MAKISHI A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2008 1 © 2008 Mónica Arakaki Makishi 2 To my parents, Bunzo and Cristina, and to my sisters and brother. 3 ACKNOWLEDGMENTS I want to express my deepest appreciation to my advisors, Douglas Soltis and Pamela Soltis, for their consistent support, encouragement and generosity of time. I would also like to thank Norris Williams and Michael Miyamoto, members of my committee, for their guidance, good disposition and positive feedback. Special thanks go to Carlos Ostolaza and Fátima Cáceres, for sharing their knowledge on Peruvian Cactaceae, and for providing essential plant material, confirmation of identifications, and their detailed observations of cacti in the field. I am indebted to the many individuals that have directly or indirectly supported me during the fieldwork: Carlos Ostolaza, Fátima Cáceres, Asunción Cano, Blanca León, José Roque, María La Torre, Richard Aguilar, Nestor Cieza, Olivier Klopfenstein, Martha Vargas, Natalia Calderón, Freddy Peláez, Yammil Ramírez, Eric Rodríguez, Percy Sandoval, and Kenneth Young (Peru); Stephan Beck, Noemí Quispe, Lorena Rey, Rosa Meneses, Alejandro Apaza, Esther Valenzuela, Mónica Zeballos, Freddy Centeno, Alfredo Fuentes, and Ramiro Lopez (Bolivia); María E. Ramírez, Mélica Muñoz, and Raquel Pinto (Chile). I thank the curators and staff of the herbaria B, F, FLAS, LPB, MO, USM, U, TEX, UNSA and ZSS, who kindly loaned specimens or made information available through electronic means. Thanks to Carlos Ostolaza for providing seeds of Haageocereus tenuis, to Graham Charles for seeds of Blossfeldia sucrensis and Acanthocalycium spiniflorum, to Donald Henne for specimens of Haageocereus lanugispinus; and to Bernard Hauser and Kent Vliet for aid with microscopy. -

Near the Himalayas, from Kashmir to Sikkim, at Altitudes the Catholic Inquisition, and the Traditional Use of These of up to 2700 Meters
Year of edition: 2018 Authors of the text: Marc Aixalà & José Carlos Bouso Edition: Alex Verdaguer | Genís Oña | Kiko Castellanos Illustrations: Alba Teixidor EU Project: New Approaches in Harm Reduction Policies and Practices (NAHRPP) Special thanks to collaborators Alejandro Ponce (in Peyote report) and Eduardo Carchedi (in Kambó report). TECHNICAL REPORT ON PSYCHOACTIVE ETHNOBOTANICALS Volumes I - II - III ICEERS International Center for Ethnobotanical Education Research and Service INDEX SALVIA DIVINORUM 7 AMANITA MUSCARIA 13 DATURA STRAMONIUM 19 KRATOM 23 PEYOTE 29 BUFO ALVARIUS 37 PSILOCYBIN MUSHROOMS 43 IPOMOEA VIOLACEA 51 AYAHUASCA 57 IBOGA 67 KAMBÓ 73 SAN PEDRO 79 6 SALVIA DIVINORUM SALVIA DIVINORUM The effects of the Hierba Pastora have been used by Mazatec Indians since ancient times to treat diseases and for divinatory purposes. The psychoactive compound Salvia divinorum contains, Salvinorin A, is the most potent naturally occurring psychoactive substance known. BASIC INFO Ska Pastora has been used in divination and healing Salvia divinorum is a perennial plant native to the Maza- rituals, similar to psilocybin mushrooms. Maria Sabina tec areas of the Sierra Madre Oriental Mountains of Mexi- told Wasson and Hofmann (the discoverers of its Mazatec co. Its habitat is tropical forests, where it grows between usage) that Salvia divinorum was used in times when the- 300 and 800 meters above sea level. It belongs to the re was a shortage of mushrooms. Some sources that have Lamiaceae family, and is mainly reproduced by cuttings done later feldwork point out that the use of S. divinorum since it rarely produces seeds. may be more widespread than originally believed, even in times when mushrooms were abundant. -
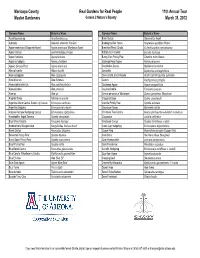
2012 Formatted Lists
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo, -

Taxonomía Y Filogenia De Trichocereus (Berg.) Riccob
UNIVERSIDAD NACIONAL DE LA PLATA FACULTAD DE CIENCIAS NATURALES Y MUSEO ―Taxonomía y filogenia de Trichocereus (Berg.) Riccob. (Trichocereeae- Cactaceae)‖ Tesis presentada para optar al grado de Doctor en Ciencias Naturales de la Universidad Nacional de La Plata Bióloga M. Sc. Adriana Sofía Albesiano Hoyos Director: Dr. Roberto Kiesling Co-Directora: Dra. Susana Freire La Plata, Argentina Agosto 3 de 2015 COMISIÓN ASESORA Dr. Roberto Kiesling, Instituto Argentino de Investigaciones de las Zonas Áridas-IADIZA, CONICET. Dra. Susana Freire, Instituto de Botánica Darwinion-San Isidro. Prof. Titular, Cátedra de Botánica Sistemática II, Facultad Ciencias Naturales y Museo, Universidad Nacional de La Plata. DEFENSA ORAL Y PÚBLICA Lugar y Fecha: Calificación: TRIBUNAL Firma:………………………………………………………...Aclaración………… Firma:………………………………………………………...Aclaración………… Firma:………………………………………………………...Aclaración………… TABLA DE CONTENIDO AGRADECIMIENTOS .............................................................................................................................. iii RESUMEN............................................................................................................................................... iv ABSTRACT ............................................................................................................................................ viii I. INTRODUCCIÓN ................................................................................................................................... 1 II. MATERIALES Y MÉTODOS ................................................................................................................ -

The Wonderful World of Cacti. July 7, 2020
OHIO STATE UNIVERSITY EXTENSION Succulents part 1: The wonderful world of cacti. July 7, 2020 Betzy Rivera. Master Gardener Volunteer OSU Extension – Franklin County OHIO STATE UNIVERSITY EXTENSION Succulent plants Are plants with parts that are thickened and fleshy, capacity that helps to retain water in arid climates. Over 25 families have species of succulents. The most representative families are: Crassulaceae, Agavaceae, Aizoaceae, Euphorbiacea and Cactaceae. 2 OHIO STATE UNIVERSITY EXTENSION The Cactaceae family is endemic to America and the distribution extends throughout the continent from Canada to Argentina, in addition to the Galapagos Islands and Antilles Most important centers of diversification (Bravo-Hollis & Sánchez-Mejorada, 1978; Hernández & Godínez, 1994; Arias-Montes, 1993; Anderson, 2001; Guzmán et al., 2003; Ortega- Baes & Godínez-Alvarez, 2006 3 OHIO STATE UNIVERSITY EXTENSION There is an exception — one of the 1,800 species occurs naturally in Africa, Sri Lanka, and Madagascar Rhipsalis baccifera 4 OHIO STATE UNIVERSITY EXTENSION The Cactaceae family includes between ~ 1,800 and 2,000 species whose life forms include climbing, epiphytic, shrubby, upright, creeping or decumbent plants, globose, cylindrical or columnar in shape (Bravo-Hollis & Sánchez-Mejorada, 1978; Hernández & Godínez, 1994; Guzmán et al., 2003). 5 OHIO STATE UNIVERSITY EXTENSION Cacti are found in a wide variety of environments, however the greatest diversity of forms is found in arid and semi-arid areas, where they play an important role in maintaining the stability of ecosystems (Bravo-Hollis & Sánchez-Mejorada, 1978; Hernández & Godínez, 1994; Guzmán et al., 2003). 6 OHIO STATE UNIVERSITY EXTENSION The Cactaceae family are dicotyledonous plants 2 cotyledons Astrophytum myriostigma (common names: Bishop´s cap cactus, bishop’s hat or miter cactus) 7 OHIO STATE UNIVERSITY EXTENSION General Anatomy of a Cactus Cactus spines are produced from specialized structures called areoles, a kind of highly reduced branch. -

Biodiversity As a Resource: Plant Use and Land Use Among the Shuar, Saraguros, and Mestizos in Tropical Rainforest Areas of Southern Ecuador
Biodiversity as a resource: Plant use and land use among the Shuar, Saraguros, and Mestizos in tropical rainforest areas of southern Ecuador Die Biodiversität als Ressource: Pflanzennutzung und Landnutzung der Shuar, Saraguros und Mestizos in tropischen Regenwaldgebieten Südecuadors Der Naturwissenschaftlichen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg zur Erlangung des Doktorgrades Dr. rer. nat. vorgelegt von Andrés Gerique Zipfel aus Valencia Als Dissertation genehmigt von der Naturwissenschaftlichen Fakultät der Friedrich-Alexander Universität Erlangen-Nürnberg Tag der mündlichen Prüfung: 9.12.2010 Vorsitzender der Promotionskommission: Prof. Dr. Rainer Fink Erstberichterstatterin: Prof. Dr. Perdita Pohle Zweitberichterstatter: Prof. Dr. Willibald Haffner To my father “He who seeks finds” (Matthew 7:8) ACKNOWLEDGEMENTS Firstly, I wish to express my gratitude to my supervisor, Prof. Dr. Perdita Pohle, for her trust and support. Without her guidance this study would not have been possible. I am especially indebted to Prof. Dr. Willibald Haffner as well, who recently passed away. His scientific knowledge and enthusiasm set a great example for me. I gratefully acknowledge Prof. Dr. Beck (Universität Bayreuth) and Prof. Dr. Knoke (Technische Universität München), and my colleagues and friends of the Institute of Geography (Friedrich-Alexander Universität Erlangen-Nürnberg) for sharing invaluable comments and motivation. Furthermore, I would like to express my sincere gratitude to those experts who unselfishly shared their knowledge with me, in particular to Dr. David Neill and Dr. Rainer Bussmann (Missouri Botanical Garden), Dr. Roman Krettek (Deutsche Gesellschaft für Mykologie), Dr. Jonathan Armbruster, (Auburn University, Alabama), Dr. Nathan K. Lujan (Texas A&M University), Dr. Jean Guffroy (Institut de Recherche pour le Développement, Orleans), Dr. -

Idpc Drug Policy Guide 3Rd Edition
IDPC DRUG POLICY GUIDE 3RD EDITION IDPC Drug Policy Guide 3 IDPC DRUG POLICY GUIDE 3RD EDITION Acknowledgements Global Drug Policy Observatory) • Dave Borden (StoptheDrugWar.org) IDPC would like to thank the following authors for drafting chapters of the 3rd Edition of the • Eric Gutierrez (Christian Aid) IDPC Drug Policy Guide: • Fabienne Hariga (United Nations Office on • Andrea Huber (Policy Director, Penal Reform Drugs and Crime) International) • George McBride (Beckley Foundation) • Benoit Gomis (Independent international • Gloria Lai (IDPC) security analyst, Associate Fellow at Chatham House, and Research Associate at Simon Fraser • Graham Bartlett (former Chief Superintendent University) of the Sussex Police) • Christopher Hallam (Research Officer, IDPC) • Gregor Burkhart (European Monitoring Centre for Drugs and Drug Addiction) • Coletta Youngers (Consultant, IDPC & Washington Office on Latin America) • Ines Gimenez • Diana Guzmán (Associate investigator, • Jamie Bridge (IDPC) DeJusticia, Associate Professor at Colombian • Javier Sagredo (United Nations Development National University and PhD candidate at Program) Stanford University) • Jean-Felix Savary (Groupement Romand • Diederik Lohman (Associate Director, Health d’Etudes en Addictologie) and Human Rights Division, Human Rights • Juan Fernandez Ochoa (IDPC) Watch) • Katherine Pettus (International Association for • Gloria Lai (Senior Policy Officer, IDPC) Hospice and Palliative Care) • Jamie Bridge (Senior Policy and Operations Manager, IDPC) • Luciana Pol (Centro de Estudios -

Cactus Explorers Journal
Bradleya 34/2016 pages 100–124 What is a cephalium? Root Gorelick Department of Biology and School of Mathematics & Statistics and Institute of Interdisciplinary Studies, Carleton University, 1125 Raven Road, Ottawa, Ontario K1S 5B6 Canada (e-mail: [email protected]) Photographs by the author unless otherwise stated. Summary : There are problems with previous at - gibt meist einen abgrenzbaren Übergang vom tempts to define ‘cephalium’, such as via produc - photosynthetisch aktiven Gewebe zum nicht pho - tion of more hairs and spines, confluence of tosynthetisch aktiven und blütentragenden areoles, or periderm development at or under - Cephalium, die beide vom gleichen Triebspitzen - neath each areole after flowering. I propose using meristem abstammen. Cephalien haben eine an - the term ‘cephalium’ only for a combination of dere Phyllotaxis als die vegetativen these criteria, i.e. flowering parts of cacti that Sprossabschnitte und sitzen der vorhandenen have confluent hairy or spiny areoles exterior to a vegetativen Phyllotaxis auf. Wenn blühende Ab - thick periderm, where these hairs, spines, and schnitte nur einen Teil der oben genannten Merk - periderms arise almost immediately below the male aufweisen, schlage ich vor, diese Strukturen shoot apical meristem, and with more hairs and als „Pseudocephalien“ zu bezeichnen. spines on reproductive parts than on photosyn - thetic parts of the shoot. Periderm development Introduction and confluent areoles preclude photosynthesis of Most cacti (Cactaceae) are peculiar plants, cephalia, which therefore lack or mostly lack even for angiosperms, with highly succulent stomata. There is almost always a discrete tran - stems, numerous highly lignified leaves aka sition from photosynthetic vegetative tissues to a spines, lack of functional photosynthetic leaves, non-photosynthetic flower-bearing cephalium, CAM photosynthesis, huge sunken shoot apical both of which arise from the same shoot apical meristems, and fantastic stem architectures meristem. -

The Long, Strange Trip of Mescaline
BOOKS & ARTS COMMENT harmacologists gave mescaline a fair trial. In Pthe early and mid- twentieth century, it seemed more than plausible that the fash- ionable hallucinogen could be tamed into a therapeutic agent. Mescaline: A After all, it had pro- Global History found effects on the of the First human body, and had Psychedelic been used for cen- MIKE JAY turies in parts of the Yale University Press Americas as a gateway (2019) to ceremonial spiritual experience. But this psychoactive alkaloid never found its clinical indication, as science writer Mike Jay explains in Mescaline, his anthropologi- cal and medical history. In the 1950s, the attention of biomedical researchers abruptly switched to a newly synthesized molecule with similar hallucinogenic properties but fewer physical side effects: lysergic acid di ethylamide, or LSD. First synthesized by Swiss scientist Albert Hofmann in 1938, LSD went on to become a recreational drug of choice in the 1960s hippy era. And, like mescaline, it teased psychiatrists without delivering a cure. ANCIENT HISTORY Jay traces the chronology of mescaline use. The alkaloid is found in the fast-growing San Pedro cactus (Echinopsis pachanoi) that towers above the mountainous desert scrub of the Andes, and the slow-growing, ground-hugging peyote cactus (Lophophora williamsii) native to Mexico and the south- western United States. Archaeological evidence suggests that the use of these cacti in rites of long-vanished cultures goes back at least 5,000 years. Europeans first came across peyote after Spain conquered Mexico in the early six- teenth century. (It is mentioned, for instance, in a mammoth study, The General History of the Things of New Spain, begun by scholar and friar Bernardino de Sahagún in 1529.) Attempts, largely by missionaries, to suppress WISEMAN ADAM Peyote cacti at a plant nursery in Mexico. -

Crafting and Consuming an American Sonoran Desert: Global Visions, Regional Nature and National Meaning
Crafting and Consuming an American Sonoran Desert: Global Visions, Regional Nature and National Meaning Item Type text; Electronic Dissertation Authors Burtner, Marcus Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 02/10/2021 04:13:17 Link to Item http://hdl.handle.net/10150/268613 CRAFTING AND CONSUMING AN AMERICAN SONORAN DESERT: GLOBAL VISIONS, REGIONAL NATURE AND NATIONAL MEANING by Marcus Alexander Burtner ____________________________________ copyright © Marcus Alexander Burtner 2012 A Dissertation Submitted to the Faculty of the DEPARTMENT OF HISTORY In Partial Fulfillment of the Requirements for the degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2012 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Marcus A. Burtner entitled “Crafting and Consuming an American Sonoran Desert: Global Visions, Regional Nature, and National Meaning.” and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy ____________________________________________________________Date: 1/7/13 Katherine Morrissey ____________________________________________________________Date: 1/7/13 Douglas Weiner ____________________________________________________________Date: 1/7/13 Jeremy Vetter ____________________________________________________________Date: 1/7/13 Jack C. Mutchler Final approval and acceptance of this dissertation is contingent upon the candidate's submission of the final copies of the dissertation to the Graduate College. I hereby certify that I have read this dissertation prepared under my direction and recommend that it be accepted as fulfilling the dissertation requirement. -

Hout Vrienden Club LIGNUM
Hout Vrienden club LIGNUM Description L 007 Lignum No: 007 : Peruvian Pillar Cactus Echinopsis pachanoi (Britton &H.Friedrich & G.D. Rowley, syn. Trichocereus peruvianus Britton & Rose. Cactaceae). Local names : Cactus de San Pedro, Huachuma, Wachuma, Aguacolla Family : Cactaceae Natural distribution area : Peru Origoin of Specimen : Armenoi Apokoronou, Chania, Crete, Greece Weight by volume : approx. 400 kg/M3, tested on samples Date : September, 2018 Provided by : Willem Hurkmans Greyred discolorations in the wood Peruvian Pillar Cactus Echinopsis pachanoi in the isle of Crete General information Echinopsis comprises about 150 described species and is a large genus within the Cactyoideae, lim- ited to South America. The distinction between E. pachanoi and E. peruvianus appears to be difficult; some botanists consider them forms of the same species while others consider them to be distinct species. Date: April, 2019 Hout Vrienden club LIGNUM Habitat Echinopsis pachanoi grows at an alti- tude of 1000-3000 m asl in the Andes mountains of Peru in semi-arid areas where at night it can be cold. Flowering (mature) specimens can be from 3 to 7 metres high and can show numerous side branches. Flowers of Echinopsis pachanoi on a cutting of the same specimen that yielded the wood specimens! Since this cactus contains from 0,3% up to 1,2% of mescaline it's a psychotropic plant, the use of which can cause an altered state of consciousness. Don't try this at home. Medicinal use of E. pacanoi was already described 3,000 years ago, for example within the Chavin culture. E. pachanoi is alleged to be effective in case of high blood pressure and cardio-vascular diseases.