February 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mapping the Dissociated Body
Lesley University DigitalCommons@Lesley Graduate School of Arts and Social Sciences Expressive Therapies Capstone Theses (GSASS) Spring 5-16-2020 Mapping the Dissociated Body Elizabeth Hough [email protected] Follow this and additional works at: https://digitalcommons.lesley.edu/expressive_theses Part of the Social and Behavioral Sciences Commons Recommended Citation Hough, Elizabeth, "Mapping the Dissociated Body" (2020). Expressive Therapies Capstone Theses. 239. https://digitalcommons.lesley.edu/expressive_theses/239 This Thesis is brought to you for free and open access by the Graduate School of Arts and Social Sciences (GSASS) at DigitalCommons@Lesley. It has been accepted for inclusion in Expressive Therapies Capstone Theses by an authorized administrator of DigitalCommons@Lesley. For more information, please contact [email protected], [email protected]. Running Head: MAPPING THE DISSOCIATED BODY 1 Mapping the Dissociated Body Elizabeth Hough Lesley University Running Head: MAPPING THE DISSOCIATED BODY 2 Abstract This capstone thesis explored the use of body mapping and body scans as a tool for assessing and tracking somatic dissociation and embodiment. The researcher utilized a client- centered approach and mindfulness-based interventions and theory to ground the work with the clients. While there were a variety of questionnaire-based tools for assessing dissociation with clients, many of them were lacking in the somatic component of dissociation. The available assessments were also exclusively self-reported and written or verbal, which had the potential to result in biased reporting. Clients may have also struggled to identify their level of somatic dissociation due to an inherent disconnection or dismissal of their somatic experience. This research described two case studies in which body scans and body mapping were utilized as a method to assess and track the client’s level of body dissociation and embodiment. -

Cactus Chronicle
Volume 78 Issue 8 HolidayCACTUS Party CHRONICLE August 2013 Mission Statement: Plant of the Month The Los Angeles Cactus and Succulent Society (LACSS) cultivates the study and enjoyment Stenocactus Bursera, of cacti and succulent plants through educational programs and activities that promote the Commiphora hobby within a community of fellow enthusiasts and among the greater public. Refreshments Our next general meeting is Letters U-Z August 1 July New Members Pat Byrne Janice B. Lee Program Title: The Exotic Fauna and Flora of East Africa Lynn Ruger Anika Russell Lauren Stanton Presented by Steve Frieze Sonia Villarroel Editor Phyllis Frieze [email protected] Visit Us on the web http:// www.lacss.com Steve Frieze is a past president of the LACSS and has served the club in numerous other capac- ities during the past 25 years he has been a member. He is currently a partner of Desert Crea- tions, a plant store that sells unusual cacti and succulents. He has traveled to numerous loca- tions that house exotic plants, including Chile, Brazil, and Oaxaca Mexico which he just returned from. This presentation will concentrate on a trip to Tanzania he took three years ago and the wonderful succulent plants and animals that are endemic to this geographic location. You will have an opportunity to see specimen size plants such as Adenias and Commiphoras that over- whelm the senses. In addition, you will be exposed to fauna that you, in many cases, could reach out and touch if you had a touch of insanity. In one instance his tour stumbled across a fully grown lion sleeping the roadway no more than five feet from the vehicle that he was sitting in. -

Hallucinogens - LSD, Peyote, Psilocybin, and PCP
Hallucinogens - LSD, Peyote, Psilocybin, and PCP Hallucinogenic compounds found in some • Psilocybin (4-phosphoryloxy-N,N- plants and mushrooms (or their extracts) dimethyltryptamine) is obtained from have been used—mostly during religious certain types of mushrooms that are rituals—for centuries. Almost all indigenous to tropical and subtropical hallucinogens contain nitrogen and are regions of South America, Mexico, and classified as alkaloids. Many hallucinogens the United States. These mushrooms have chemical structures similar to those of typically contain less than 0.5 percent natural neurotransmitters (e.g., psilocybin plus trace amounts of acetylcholine-, serotonin-, or catecholamine- psilocin, another hallucinogenic like). While the exact mechanisms by which substance. hallucinogens exert their effects remain • PCP (phencyclidine) was developed in unclear, research suggests that these drugs the 1950s as an intravenous anesthetic. work, at least partially, by temporarily Its use has since been discontinued due interfering with neurotransmitter action or to serious adverse effects. by binding to their receptor sites. This DrugFacts will discuss four common types of How Are Hallucinogens Abused? hallucinogens: The very same characteristics that led to • LSD (d-lysergic acid diethylamide) is the incorporation of hallucinogens into one of the most potent mood-changing ritualistic or spiritual traditions have also chemicals. It was discovered in 1938 led to their propagation as drugs of abuse. and is manufactured from lysergic acid, Importantly, and unlike most other drugs, which is found in ergot, a fungus that the effects of hallucinogens are highly grows on rye and other grains. variable and unreliable, producing different • Peyote is a small, spineless cactus in effects in different people at different times. -

Hallucinogens - LSD, Peyote, Psilocybin, and PCP
Information for Behavioral Health Providers in Primary Care Hallucinogens - LSD, Peyote, Psilocybin, and PCP What are Hallucinogens? Hallucinogenic compounds found in some plants and mushrooms (or their extracts) have been used— mostly during religious rituals—for centuries. Almost all hallucinogens contain nitrogen and are classified as alkaloids. Many hallucinogens have chemical structures similar to those of natural neurotransmitters (e.g., acetylcholine-, serotonin-, or catecholamine-like). While the exact mechanisms by which hallucinogens exert their effects remain unclear, research suggests that these drugs work, at least partially, by temporarily interfering with neurotransmitter action or by binding to their receptor sites. This InfoFacts will discuss four common types of hallucinogens: LSD (d-lysergic acid diethylamide) is one of the most potent mood-changing chemicals. It was discovered in 1938 and is manufactured from lysergic acid, which is found in ergot, a fungus that grows on rye and other grains. Peyote is a small, spineless cactus in which the principal active ingredient is mescaline. This plant has been used by natives in northern Mexico and the southwestern United States as a part of religious ceremonies. Mescaline can also be produced through chemical synthesis. Psilocybin (4-phosphoryloxy-N, N-dimethyltryptamine) is obtained from certain types of mushrooms that are indigenous to tropical and subtropical regions of South America, Mexico, and the United States. These mushrooms typically contain less than 0.5 percent psilocybin plus trace amounts of psilocin, another hallucinogenic substance. PCP (phencyclidine) was developed in the 1950s as an intravenous anesthetic. Its use has since been discontinued due to serious adverse effects. How Are Hallucinogens Abused? The very same characteristics that led to the incorporation of hallucinogens into ritualistic or spiritual traditions have also led to their propagation as drugs of abuse. -

Hallucinogens and Dissociative Drugs
Long-Term Effects of Hallucinogens See page 5. from the director: Research Report Series Hallucinogens and dissociative drugs — which have street names like acid, angel dust, and vitamin K — distort the way a user perceives time, motion, colors, sounds, and self. These drugs can disrupt a person’s ability to think and communicate rationally, or even to recognize reality, sometimes resulting in bizarre or dangerous behavior. Hallucinogens such as LSD, psilocybin, peyote, DMT, and ayahuasca cause HALLUCINOGENS AND emotions to swing wildly and real-world sensations to appear unreal, sometimes frightening. Dissociative drugs like PCP, DISSOCIATIVE DRUGS ketamine, dextromethorphan, and Salvia divinorum may make a user feel out of Including LSD, Psilocybin, Peyote, DMT, Ayahuasca, control and disconnected from their body PCP, Ketamine, Dextromethorphan, and Salvia and environment. In addition to their short-term effects What Are on perception and mood, hallucinogenic Hallucinogens and drugs are associated with psychotic- like episodes that can occur long after Dissociative Drugs? a person has taken the drug, and dissociative drugs can cause respiratory allucinogens are a class of drugs that cause hallucinations—profound distortions depression, heart rate abnormalities, and in a person’s perceptions of reality. Hallucinogens can be found in some plants and a withdrawal syndrome. The good news is mushrooms (or their extracts) or can be man-made, and they are commonly divided that use of hallucinogenic and dissociative Hinto two broad categories: classic hallucinogens (such as LSD) and dissociative drugs (such drugs among U.S. high school students, as PCP). When under the influence of either type of drug, people often report rapid, intense in general, has remained relatively low in emotional swings and seeing images, hearing sounds, and feeling sensations that seem real recent years. -

Hallucinogens
Hallucinogens What Are Hallucinogens? Hallucinogens are a diverse group of drugs that alter a person’s awareness of their surroundings as well as their thoughts and feelings. They are commonly split into two categories: classic hallucinogens (such as LSD) and dissociative drugs (such as PCP). Both types of hallucinogens can cause hallucinations, or sensations and images that seem real though they are not. Additionally, dissociative drugs can cause users to feel out of control or disconnected from their body and environment. Some hallucinogens are extracted from plants or mushrooms, and others are synthetic (human-made). Historically, people have used hallucinogens for religious or healing rituals. More recently, people report using these drugs for social or recreational purposes. Hallucinogens are a Types of Hallucinogens diverse group of drugs Classic Hallucinogens that alter perception, LSD (D-lysergic acid diethylamide) is one of the most powerful mind- thoughts, and feelings. altering chemicals. It is a clear or white odorless material made from lysergic acid, which is found in a fungus that grows on rye and other Hallucinogens are split grains. into two categories: Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) comes from certain classic hallucinogens and types of mushrooms found in tropical and subtropical regions of South dissociative drugs. America, Mexico, and the United States. Peyote (mescaline) is a small, spineless cactus with mescaline as its main People use hallucinogens ingredient. Peyote can also be synthetic. in a wide variety of ways DMT (N,N-dimethyltryptamine) is a powerful chemical found naturally in some Amazonian plants. People can also make DMT in a lab. -

From Sacred Plants to Psychotherapy
From Sacred Plants to Psychotherapy: The History and Re-Emergence of Psychedelics in Medicine By Dr. Ben Sessa ‘The rejection of any source of evidence is always treason to that ultimate rationalism which urges forward science and philosophy alike’ - Alfred North Whitehead Introduction: What exactly is it that fascinates people about the psychedelic drugs? And how can we best define them? 1. Most psychiatrists will define psychedelics as those drugs that cause an acute confusional state. They bring about profound alterations in consciousness and may induce perceptual distortions as part of an organic psychosis. 2. Another definition for these substances may come from the cross-cultural dimension. In this context psychedelic drugs may be recognised as ceremonial religious tools, used by some non-Western cultures in order to communicate with the spiritual world. 3. For many lay people the psychedelic drugs are little more than illegal and dangerous drugs of abuse – addictive compounds, not to be distinguished from cocaine and heroin, which are only understood to be destructive - the cause of an individual, if not society’s, destruction. 4. But two final definitions for psychedelic drugs – and those that I would like the reader to have considered by the end of this article – is that the class of drugs defined as psychedelic, can be: a) Useful and safe medical treatments. Tools that as adjuncts to psychotherapy can be used to alleviate the symptoms and course of many mental illnesses, and 1 b) Vital research tools with which to better our understanding of the brain and the nature of consciousness. Classifying psychedelic drugs: 1,2 The drugs that are often described as the ‘classical’ psychedelics include LSD-25 (Lysergic Diethylamide), Mescaline (3,4,5- trimethoxyphenylathylamine), Psilocybin (4-hydroxy-N,N-dimethyltryptamine) and DMT (dimethyltryptamine). -

XELJANZ (Tofacitinib)
HIGHLIGHTS OF PRESCRIBING INFORMATION Psoriatic Arthritis (in combination with nonbiologic DMARDs) These highlights do not include all the information needed to use XELJANZ 5 mg twice daily or XELJANZ XR 11 mg once daily. (2.2) XELJANZ/XELJANZ XR safely and effectively. See full prescribing Recommended dosage in patients with moderate and severe renal information for XELJANZ. impairment or moderate hepatic impairment is XELJANZ 5 mg once daily. (2, 8.7, 8.8) ® XELJANZ (tofacitinib) tablets, for oral use Ulcerative Colitis ® XELJANZ XR (tofacitinib) extended-release tablets, for oral use XELJANZ 10 mg twice daily for at least 8 weeks; then 5 or 10 mg Initial U.S. Approval: 2012 twice daily. Discontinue after 16 weeks of 10 mg twice daily, if adequate therapeutic benefit is not achieved. Use the lowest effective dose to WARNING: SERIOUS INFECTIONS AND MALIGNANCY maintain response. (2.3) See full prescribing information for complete boxed warning. Recommended dosage in patients with moderate and severe renal impairment or moderate hepatic impairment: half the total daily dosage Serious infections leading to hospitalization or death, including recommended for patients with normal renal and hepatic function. (2, 8.7, tuberculosis and bacterial, invasive fungal, viral, and other 8.8) opportunistic infections, have occurred in patients receiving Dosage Adjustment XELJANZ. (5.1) See the full prescribing information for dosage adjustments by indication If a serious infection develops, interrupt XELJANZ/XELJANZ XR for patients receiving CYP2C19 and/or CYP3A4 inhibitors; in patients until the infection is controlled. (5.1) with moderate or severe renal impairment or moderate hepatic Prior to starting XELJANZ/XELJANZ XR, perform a test for latent impairment; and patients with lymphopenia, neutropenia, or anemia. -

Near the Himalayas, from Kashmir to Sikkim, at Altitudes the Catholic Inquisition, and the Traditional Use of These of up to 2700 Meters
Year of edition: 2018 Authors of the text: Marc Aixalà & José Carlos Bouso Edition: Alex Verdaguer | Genís Oña | Kiko Castellanos Illustrations: Alba Teixidor EU Project: New Approaches in Harm Reduction Policies and Practices (NAHRPP) Special thanks to collaborators Alejandro Ponce (in Peyote report) and Eduardo Carchedi (in Kambó report). TECHNICAL REPORT ON PSYCHOACTIVE ETHNOBOTANICALS Volumes I - II - III ICEERS International Center for Ethnobotanical Education Research and Service INDEX SALVIA DIVINORUM 7 AMANITA MUSCARIA 13 DATURA STRAMONIUM 19 KRATOM 23 PEYOTE 29 BUFO ALVARIUS 37 PSILOCYBIN MUSHROOMS 43 IPOMOEA VIOLACEA 51 AYAHUASCA 57 IBOGA 67 KAMBÓ 73 SAN PEDRO 79 6 SALVIA DIVINORUM SALVIA DIVINORUM The effects of the Hierba Pastora have been used by Mazatec Indians since ancient times to treat diseases and for divinatory purposes. The psychoactive compound Salvia divinorum contains, Salvinorin A, is the most potent naturally occurring psychoactive substance known. BASIC INFO Ska Pastora has been used in divination and healing Salvia divinorum is a perennial plant native to the Maza- rituals, similar to psilocybin mushrooms. Maria Sabina tec areas of the Sierra Madre Oriental Mountains of Mexi- told Wasson and Hofmann (the discoverers of its Mazatec co. Its habitat is tropical forests, where it grows between usage) that Salvia divinorum was used in times when the- 300 and 800 meters above sea level. It belongs to the re was a shortage of mushrooms. Some sources that have Lamiaceae family, and is mainly reproduced by cuttings done later feldwork point out that the use of S. divinorum since it rarely produces seeds. may be more widespread than originally believed, even in times when mushrooms were abundant. -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -
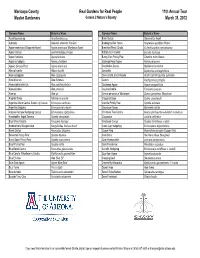
2012 Formatted Lists
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo, -

Examining the Therapist's Internal Experience When a Patient Dissociates in Session
University of Pennsylvania ScholarlyCommons Doctorate in Social Work (DSW) Dissertations School of Social Policy and Practice Spring 5-13-2013 Do You Know What I Know? Examining the Therapist's Internal Experience when a Patient Dissociates in Session Jacqueline R. Strait University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations_sp2 Part of the Psychology Commons, and the Social Work Commons Recommended Citation Strait, Jacqueline R., "Do You Know What I Know? Examining the Therapist's Internal Experience when a Patient Dissociates in Session" (2013). Doctorate in Social Work (DSW) Dissertations. 36. https://repository.upenn.edu/edissertations_sp2/36 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations_sp2/36 For more information, please contact [email protected]. Do You Know What I Know? Examining the Therapist's Internal Experience when a Patient Dissociates in Session Abstract There is rich theoretical literature that cites the importance of the therapist’s use of self as a way of knowing, especially in cases where a patient has been severely traumatized in early life. There is limited empirical research that explores the in-session experience of therapists working with traumatized patients in order to support these claims. This study employed a qualitative design to explore a therapist’s internal experience when a patient dissociates in session. The aim of this study was to further develop the theoretical construct of dissociative attunement to explain the way that therapist and patient engage in a nonverbal process of synchronicity that has the potential to communicate dissociated images, affect or somatosensory experiences by way of the therapist’s internal experience.