Supplementary Information For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cactus Chronicle
Volume 78 Issue 8 HolidayCACTUS Party CHRONICLE August 2013 Mission Statement: Plant of the Month The Los Angeles Cactus and Succulent Society (LACSS) cultivates the study and enjoyment Stenocactus Bursera, of cacti and succulent plants through educational programs and activities that promote the Commiphora hobby within a community of fellow enthusiasts and among the greater public. Refreshments Our next general meeting is Letters U-Z August 1 July New Members Pat Byrne Janice B. Lee Program Title: The Exotic Fauna and Flora of East Africa Lynn Ruger Anika Russell Lauren Stanton Presented by Steve Frieze Sonia Villarroel Editor Phyllis Frieze [email protected] Visit Us on the web http:// www.lacss.com Steve Frieze is a past president of the LACSS and has served the club in numerous other capac- ities during the past 25 years he has been a member. He is currently a partner of Desert Crea- tions, a plant store that sells unusual cacti and succulents. He has traveled to numerous loca- tions that house exotic plants, including Chile, Brazil, and Oaxaca Mexico which he just returned from. This presentation will concentrate on a trip to Tanzania he took three years ago and the wonderful succulent plants and animals that are endemic to this geographic location. You will have an opportunity to see specimen size plants such as Adenias and Commiphoras that over- whelm the senses. In addition, you will be exposed to fauna that you, in many cases, could reach out and touch if you had a touch of insanity. In one instance his tour stumbled across a fully grown lion sleeping the roadway no more than five feet from the vehicle that he was sitting in. -

Lectotypification of Banisteriopsis Caapi and B. Quitensis
________________________________________________________________________________________________www.neip.info TAXON 00 (00) • 1–4 Oliveira & al. • Lectotypification of Banisteriopsis caapi NOMENCLATURE Lectotypification of Banisteriopsis caapi and B. quitensis (Malpighiaceae), names associated with an important ingredient of Ayahuasca Regina Célia de Oliveira,1 Júlia Sonsin-Oliveira,1 Thaís Aparecida Coelho dos Santos,1 Marcelo Simas e Silva,2 Christopher William Fagg1 & Renata Sebastiani3 1 Programa de Pós-Graduação (PPG) em Botânica, Departamento de Botânica, Instituto de Ciências Biológicas (IB), Universidade de Brasília (UnB), Brasília, DF, 70919-970, Brazil 2 Rio de Janeiro, Brazil (Independent Researcher) 3 PPG em Ciências Ambientais, Universidade Federal de São Carlos, Rodovia Anhanguera, km 174, CP 153, Araras, São Paulo, 13600-970, Brazil Address for correspondence: Regina C. Oliveira, [email protected] DOI https://doi.org/10.1002/tax.12407 Abstract Ritually used in religious ceremonies and now popular culture, Banisteriopsis caapi (≡ Banisteria caapi) is the most impor- tant ingredient in an inebriating drink known as Ayahuasca. The nomenclatural history of B. caapi and B. quitensis is presented, and both names are lectotypified. Keywords Ayahuasca; Banisteria; Daime; entheogen; Hoasca; vegetal; Yagé ■ INTRODUCTION While botanists treat the vine used in Ayahuasca as com- prising of either one or two species, those who traditionally The Malpighiaceae is principally a tropical family, cur- use it recognize multiple entities or kinds, here referred to as rently with ~1300 species in 77 genera accepted in the New variants for the sake of simplicity (e.g., Spruce, 1908; Koch- World and ~150 species belonging to 17 genera exclusively Grunberg, 1923; Gates, 1982; Langdon, 1986; Schultes, 1986; in the Old World (Davis & Anderson, 2010). -

Plant Classification, Evolution and Reproduction
Plant Classification, Evolution, and Reproduction Plant classification, evolution and reproduction! Traditional plant classification! ! A phylogenetic perspective on classification! ! Milestones of land plant evolution! ! Overview of land plant diversity! ! Life cycle of land plants! Classification “the ordering of diversity into a meaningful hierarchical pattern” (i.e., grouping)! The Taxonomic Hierarchy! Classification of Ayahuasca, Banisteriopsis caapi! Kingdom !Plantae! Phylum !Magnoliophyta Class ! !Magnoliopsida! Order !Malpighiales! Family !Malpighiaceae Genus ! !Banisteriopsis! Species !caapi! Ranks above genus have standard endings.! Higher categories are more inclusive.! Botanical nomenclature Carolus Linnaeus (1707–1778)! Species Plantarum! published 1753! 7,300 species! Botanical nomenclature Polynomials versus binomials! Know the organism “The Molesting Salvinia” Salvinia auriculata (S. molesta)! hp://dnr.state.il.us/stewardship/cd/biocontrol/2floangfern.html " Taxonomy vs. classification! Assigning a name! A system ! ! ! Placement in a category! Often predictive ! because it is based on Replicable, reliable relationships! results! ! Relationships centered on genealogy ! ! ! ! Edward Hitchcock, Elementary Geology, 1940! Classification Phylogeny: Reflect hypothesized evolution. relationships! Charles Darwin, Origin of Species, 1859! Ernst Haeckel, Generelle Morphologie der Organismen, 1866! Branching tree-like diagrams representing relationships! Magnolia 1me 2 Zi m merman (1930) Lineage branching (cladogenesis or speciation) Modified -

The Genus Anadenanthera in Amerindian Cultures
THE GENUS ANADENANTHERA IN AMERINDIAN CULTURES BY SIRI VON REIS ALTSCHUL, PH .D. RESEARCH FELLOW BOTANICAL MUSEUM HARVARD UNIVERSITY CAMBRIDGE, MASSACHUSETTS 1972 This monograph is dedicated in affectionate memory to the late DANIEL HERBERT EFRON, PH.D., M.D. 1913-1972 cherished friend of the author and of the Botanical Museum, a true scientist devoted to the interdisciplinary approach in the advancement of knowledge. A/""'f""'<J liz {i>U<// t~ },~u 0<<J4 ~ If;. r:J~ ~ //"'~uI ~ ()< d~ ~ !dtd't;:..1 "./.u.L .A Vdl0 If;;: ~ '" OU'''-k4 :/" tu-d ''''''"-''t2.. ?,,".jd,~ jft I;ft'- ?_rl; A~~ ~r'4tft,t -5 " q,.,<,4 ~~ l' #- /""/) -/~ "1'Ii;. ,1""", "/'/1'1",, I X C"'-r'fttt. #) (../..d ~;, . W,( ~ ~ f;r"'" y it;.,,J 11/" Y 4J.. %~~ l{jr~ t> ~~ ~txh '1'ix r 4 6~" c/<'T'''(''-;{' rn« ?.d ~;;1';;/ a-.d txZ-~ ~ ;o/~ <A.H-iz "" ~".,/( 1-/X< "..< ,:" -.... ~ ~ . JJlr-0? on . .it-(,0.1' r 4 -11<.1.- aw./{') -:JL. P7t;;"j~;1 S .d-At ;0~/lAQ<..t ,ti~?,f,.... vj "7rU<-'- ~I""" =iiR-I1;M~ a....k«<-l, ¢- f!!) d..;.:~ M ~ ~y£/1 ~/.u..-... It'--, "" # :Z:-,k. "i ~ "d/~ efL<.<~/ ,w 1'#,') /';~~;d-t a;.. tlArl-<7'" I .Ii;'~.1 (1(-;.,} >Lc -(l"7C),.,..,;.. :.... ,,:/ ~ /-V,~ , ,1" # (i F'"' l' fJ~~A- (.tG- ~/~ Z:--7Co- ,,:. ,L7r= f,-, , ~t) ahd-p;: fJ~ / tr>d .4 ~f- $. b".,,1 ~/. ~ pd. 1'7'-· X ~-t;;;;.,~;z jM ~0Y:tJ;; ~ """.,4? br;K,' ./.n.u" ~ 7r .".,.~,j~ ;;f;tT ~ ..4'./ ;pf,., tJd~ M_~ (./I<'/~.'. IU. et. c./,. ~L.y !f-t.<H>:t;.tu ~ ~,:,-,p., .....:. -

The Alkaloids of Banisteriopsis Caapi, the Plant Source of the Amazonian
www.nature.com/scientificreports OPEN The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Received: 16 March 2017 Accepted: 7 June 2017 Ayahuasca, stimulate adult Published: xx xx xxxx neurogenesis in vitro Jose A. Morales-García 1,2,3, Mario de la Fuente Revenga 4,5,8, Sandra Alonso-Gil1,2, María Isabel Rodríguez-Franco5, Amanda Feilding6, Ana Perez-Castillo1,2 & Jordi Riba4,7 Banisteriopsis caapi is the basic ingredient of ayahuasca, a psychotropic plant tea used in the Amazon for ritual and medicinal purposes, and by interested individuals worldwide. Animal studies and recent clinical research suggests that B. caapi preparations show antidepressant activity, a therapeutic efect that has been linked to hippocampal neurogenesis. Here we report that harmine, tetrahydroharmine and harmaline, the three main alkaloids present in B. caapi, and the harmine metabolite harmol, stimulate adult neurogenesis in vitro. In neurospheres prepared from progenitor cells obtained from the subventricular and the subgranular zones of adult mice brains, all compounds stimulated neural stem cell proliferation, migration, and diferentiation into adult neurons. These fndings suggest that modulation of brain plasticity could be a major contribution to the antidepressant efects of ayahuasca. They also expand the potential application of B. caapi alkaloids to other brain disorders that may beneft from stimulation of endogenous neural precursor niches. Ayahuasca is the Quechua name used to designate Banisteriopsis caapi, a jungle liana of the Malpighiaceae family that is native to the Amazon and Orinoco river basins1. Te term is also applied to the tea that is obtained by infusing in water the stems of B. -

(DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact
pharmaceuticals Review Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact Andreia Machado Brito-da-Costa 1 , Diana Dias-da-Silva 1,2,* , Nelson G. M. Gomes 1,3 , Ricardo Jorge Dinis-Oliveira 1,2,4,* and Áurea Madureira-Carvalho 1,3 1 Department of Sciences, IINFACTS-Institute of Research and Advanced Training in Health Sciences and Technologies, University Institute of Health Sciences (IUCS), CESPU, CRL, 4585-116 Gandra, Portugal; [email protected] (A.M.B.-d.-C.); ngomes@ff.up.pt (N.G.M.G.); [email protected] (Á.M.-C.) 2 UCIBIO-REQUIMTE, Laboratory of Toxicology, Department of Biological Sciences, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal 3 LAQV-REQUIMTE, Laboratory of Pharmacognosy, Department of Chemistry, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal 4 Department of Public Health and Forensic Sciences, and Medical Education, Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal * Correspondence: [email protected] (D.D.-d.-S.); [email protected] (R.J.D.-O.); Tel.: +351-224-157-216 (R.J.D.-O.) Received: 21 September 2020; Accepted: 20 October 2020; Published: 23 October 2020 Abstract: Ayahuasca is a hallucinogenic botanical beverage originally used by indigenous Amazonian tribes in religious ceremonies and therapeutic practices. While ethnobotanical surveys still indicate its spiritual and medicinal uses, consumption of ayahuasca has been progressively related with a recreational purpose, particularly in Western societies. The ayahuasca aqueous concoction is typically prepared from the leaves of the N,N-dimethyltryptamine (DMT)-containing Psychotria viridis, and the stem and bark of Banisteriopsis caapi, the plant source of harmala alkaloids. -
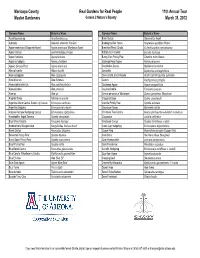
2012 Formatted Lists
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo, -

The Evolutionary Fate of Rpl32 and Rps16 Losses in the Euphorbia Schimperi (Euphorbiaceae) Plastome Aldanah A
www.nature.com/scientificreports OPEN The evolutionary fate of rpl32 and rps16 losses in the Euphorbia schimperi (Euphorbiaceae) plastome Aldanah A. Alqahtani1,2* & Robert K. Jansen1,3 Gene transfers from mitochondria and plastids to the nucleus are an important process in the evolution of the eukaryotic cell. Plastid (pt) gene losses have been documented in multiple angiosperm lineages and are often associated with functional transfers to the nucleus or substitutions by duplicated nuclear genes targeted to both the plastid and mitochondrion. The plastid genome sequence of Euphorbia schimperi was assembled and three major genomic changes were detected, the complete loss of rpl32 and pseudogenization of rps16 and infA. The nuclear transcriptome of E. schimperi was sequenced to investigate the transfer/substitution of the rpl32 and rps16 genes to the nucleus. Transfer of plastid-encoded rpl32 to the nucleus was identifed previously in three families of Malpighiales, Rhizophoraceae, Salicaceae and Passiforaceae. An E. schimperi transcript of pt SOD-1- RPL32 confrmed that the transfer in Euphorbiaceae is similar to other Malpighiales indicating that it occurred early in the divergence of the order. Ribosomal protein S16 (rps16) is encoded in the plastome in most angiosperms but not in Salicaceae and Passiforaceae. Substitution of the E. schimperi pt rps16 was likely due to a duplication of nuclear-encoded mitochondrial-targeted rps16 resulting in copies dually targeted to the mitochondrion and plastid. Sequences of RPS16-1 and RPS16-2 in the three families of Malpighiales (Salicaceae, Passiforaceae and Euphorbiaceae) have high sequence identity suggesting that the substitution event dates to the early divergence within Malpighiales. -

Bifurcated Snuff Tubes in the Pre-Columbian Caribbean
Journal of Caribbean Archaeology Copyright 2020 ISBN 1524-4776 Conduits to the supernatural: Bifurcated snuff tubes in the pre-Columbian Caribbean Joanna Ostapkowicz School of Archaeology University of Oxford Oxford, OX1 2PG, UK [email protected] UK museum collections hold an important and largely unexplored corpus of Caribbean pre-Columbian cultural heritage, including seminal pieces that can offer new insights into the development of complex rituals in the region. This paper re-establishes the cultural context and significance of a previously undocumented carving related to cohoba drug rituals: an ornate, composite snuff tube carved of cannel coal, recovered from the Lesser Antillean island of St Vincent before 1870, and donated to Oxford's Pitt Rivers Museum in 1900. Both the material (which does not occur in the insular Caribbean) and the carving style suggest that the snuff tube was an import from Venezuela's Lower Orinoco region, where the Barrancoid style emerged in its classic form ca. ~100 BC - AD 500 (Los Barrancos complex). As such, it is the earliest example of drug paraphernalia often assumed to have been used only after ~AD 1000, and isolated to the chiefdom-level societies of the Greater Antilles. This paper contributes a brief review of the St Vincent snuff tube within the context of other stone and wood examples in public collections in efforts to explore their diagnostics, range and, ultimately, the ceremonies in which they were used. Las colecciones museísticas del Reino Unido conservan un importante y a su vez poco conocido corpus de materiales pertenecientes al patrimonio precolombino caribeño, entre los que se incluyen piezas destacadas que pueden ofrecer información sobre el desarrollo de rituales complejos en la región. -

Inner Visions: Sacred Plants, Art and Spirituality
AM 9:31 2 12/10/14 2 224926_Covers_DEC10.indd INNER VISIONS: SACRED PLANTS, ART AND SPIRITUALITY Brauer Museum of Art • Valparaiso University Vision 12: Three Types of Sorcerers Gouache on paper, 12 x 16 inches. 1989 Pablo Amaringo 224926_Covers_DEC10.indd 3 12/10/14 9:31 AM 3 224926_Text_Dec12.indd 3 12/12/14 11:42 AM Inner Visions: Sacred Plants, Art and Spirituality • An Exhibition of Art Presented by the Brauer Museum • Curated by Luis Eduardo Luna 4 224926_Text.indd 4 12/9/14 10:00 PM Contents 6 From the Director Gregg Hertzlieb 9 Introduction Robert Sirko 13 Inner Visions: Sacred Plants, Art and Spirituality Luis Eduardo Luna 29 Encountering Other Worlds, Amazonian and Biblical Richard E. DeMaris 35 The Artist and the Shaman: Seen and Unseen Worlds Robert Sirko 73 Exhibition Listing 5 224926_Text.indd 5 12/9/14 10:00 PM From the Director In this Brauer Museum of Art exhibition and accompanying other than earthly existence. Additionally, while some objects publication, expertly curated by the noted scholar Luis Eduardo may be culture specific in their references and nature, they are Luna, we explore the complex and enigmatic topic of the also broadly influential on many levels to, say, contemporary ritual use of sacred plants to achieve visionary states of mind. American and European subcultures, as well as to contemporary Working as a team, Luna, Valparaiso University Associate artistic practices in general. Professor of Art Robert Sirko, Valparaiso University Professor We at the Brauer Museum of Art wish to thank the Richard E. DeMaris and the Brauer Museum staff present following individuals and agencies for making this exhibition our efforts of examining visual products arising from the possible: the Brauer Museum of Art’s Brauer Endowment, ingestion of these sacred plants and brews such as ayahuasca. -

Biodiversity As a Resource: Plant Use and Land Use Among the Shuar, Saraguros, and Mestizos in Tropical Rainforest Areas of Southern Ecuador
Biodiversity as a resource: Plant use and land use among the Shuar, Saraguros, and Mestizos in tropical rainforest areas of southern Ecuador Die Biodiversität als Ressource: Pflanzennutzung und Landnutzung der Shuar, Saraguros und Mestizos in tropischen Regenwaldgebieten Südecuadors Der Naturwissenschaftlichen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg zur Erlangung des Doktorgrades Dr. rer. nat. vorgelegt von Andrés Gerique Zipfel aus Valencia Als Dissertation genehmigt von der Naturwissenschaftlichen Fakultät der Friedrich-Alexander Universität Erlangen-Nürnberg Tag der mündlichen Prüfung: 9.12.2010 Vorsitzender der Promotionskommission: Prof. Dr. Rainer Fink Erstberichterstatterin: Prof. Dr. Perdita Pohle Zweitberichterstatter: Prof. Dr. Willibald Haffner To my father “He who seeks finds” (Matthew 7:8) ACKNOWLEDGEMENTS Firstly, I wish to express my gratitude to my supervisor, Prof. Dr. Perdita Pohle, for her trust and support. Without her guidance this study would not have been possible. I am especially indebted to Prof. Dr. Willibald Haffner as well, who recently passed away. His scientific knowledge and enthusiasm set a great example for me. I gratefully acknowledge Prof. Dr. Beck (Universität Bayreuth) and Prof. Dr. Knoke (Technische Universität München), and my colleagues and friends of the Institute of Geography (Friedrich-Alexander Universität Erlangen-Nürnberg) for sharing invaluable comments and motivation. Furthermore, I would like to express my sincere gratitude to those experts who unselfishly shared their knowledge with me, in particular to Dr. David Neill and Dr. Rainer Bussmann (Missouri Botanical Garden), Dr. Roman Krettek (Deutsche Gesellschaft für Mykologie), Dr. Jonathan Armbruster, (Auburn University, Alabama), Dr. Nathan K. Lujan (Texas A&M University), Dr. Jean Guffroy (Institut de Recherche pour le Développement, Orleans), Dr. -

Idpc Drug Policy Guide 3Rd Edition
IDPC DRUG POLICY GUIDE 3RD EDITION IDPC Drug Policy Guide 3 IDPC DRUG POLICY GUIDE 3RD EDITION Acknowledgements Global Drug Policy Observatory) • Dave Borden (StoptheDrugWar.org) IDPC would like to thank the following authors for drafting chapters of the 3rd Edition of the • Eric Gutierrez (Christian Aid) IDPC Drug Policy Guide: • Fabienne Hariga (United Nations Office on • Andrea Huber (Policy Director, Penal Reform Drugs and Crime) International) • George McBride (Beckley Foundation) • Benoit Gomis (Independent international • Gloria Lai (IDPC) security analyst, Associate Fellow at Chatham House, and Research Associate at Simon Fraser • Graham Bartlett (former Chief Superintendent University) of the Sussex Police) • Christopher Hallam (Research Officer, IDPC) • Gregor Burkhart (European Monitoring Centre for Drugs and Drug Addiction) • Coletta Youngers (Consultant, IDPC & Washington Office on Latin America) • Ines Gimenez • Diana Guzmán (Associate investigator, • Jamie Bridge (IDPC) DeJusticia, Associate Professor at Colombian • Javier Sagredo (United Nations Development National University and PhD candidate at Program) Stanford University) • Jean-Felix Savary (Groupement Romand • Diederik Lohman (Associate Director, Health d’Etudes en Addictologie) and Human Rights Division, Human Rights • Juan Fernandez Ochoa (IDPC) Watch) • Katherine Pettus (International Association for • Gloria Lai (Senior Policy Officer, IDPC) Hospice and Palliative Care) • Jamie Bridge (Senior Policy and Operations Manager, IDPC) • Luciana Pol (Centro de Estudios