Benzocaine: Summary Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Hygroscopicity of Pharmaceutical Crystals
HYGROSCOPICITY OF PHARMACEUTICAL CRYSTALS A DISSERTATION SUBMITTED TO THE FACULTY OF GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY DABING CHEN IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY RAJ SURYANARAYANAN (ADVISER) JANUARY, 2009 © Dabing Chen, January / 2009 ACKNOWLEDGEMENTS I am very grateful to my thesis advisor, Prof. Raj Suryanarayanan, for his constant guidance, support, and encouragement throughout my research. Without his help, the completion of this thesis would be impossible. His friendship and advices are precious to my professional and personal growth and will help me overcome many difficulties in my future career. I would like to take the opportunity to thank Prof. David J.W. Grant, who was my advisor during the first three years in graduate school and led me into the research area of physical pharmacy. It was my great honor to have worked for him, and he will always live as a role model in my life. Many thanks to Dr. Zheng Jane Li at Boehringer Ingelheim Pharmaceuticals (BI) for her invaluable advice as an industrial mentor and also for agreeing to serve on my committee. I sincerely appreciate her helpful discussions, revision of the manuscripts, and supervision of my research. I also want to thank her for providing me the internship opportunity at BI. I thank Dr. Timothy S. Wiedmann and Dr. Theodore P. Labuza for serving on my committee and for critically reviewing my thesis. I also want to thank Dr. Timothy S. Wiedmann for allowing me the use of the HPLC instruments in his lab and also for his advice as the Director of Graduate Studies. -

Hyperinflation Management Medications Requiring Prior Authorization for Medical Necessity
July 2021 Effective 07/01/2021 Hyperinflation Management Medications Requiring Prior Authorization for Medical Necessity Below is a list of medicines by drug class that will not be covered without a prior authorization for medical necessity. If you continue using one of these drugs without prior approval for medical necessity, you may be required to pay the full cost. If you are currently using one of the drugs requiring prior authorization for medical necessity, ask your d octor to choose one of the generic or brand formulary options listed below. Category * Drugs Requiring Prior Formulary Options Drug Class Authorization for Medical Necessity 1 Allergies Dexchlorpheniramine levocetirizine Antihistamines Diphen Elixir (NDC^ 69067009204 only) RyClora CARBINOXAMINE TABLET 6 MG Anti-convulsants topiramate ext-rel capsule carbamazepine, carbamazepine ext-rel, (generics for QUDEXY XR only) clobazam, divalproex sodium, divalproex sodium ext-rel, gabapentin, lamotrigine, lamotrigine ext-rel, levetiracetam, levetiracetam ext-rel, oxcarbazepine, phenobarbital, phenytoin, phenytoin sodium extended, primidone, rufinamide, tiagabine, topiramate, valproic acid, zonisamide, FYCOMPA, OXTELLAR XR, TROKENDI XR, VIMPAT, XCOPRI ZONEGRAN carbamazepine, carbamazepine ext-rel, divalproex sodium, divalproex sodium ext-rel, gabapentin, lamotrigine, lamotrigine ext-rel, levetiracetam, levetiracetam ext-rel, oxcarbazepine, phenobarbital, phenytoin, phenytoin sodium extended, primidone, tiagabine, topiramate, valproic acid, zonisamide, FYCOMPA, OXTELLAR XR, TROKENDI -

Ep 1931310 B1
(19) & (11) EP 1 931 310 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/12 (2006.01) A61K 9/06 (2006.01) 06.06.2012 Bulletin 2012/23 A61K 9/70 (2006.01) A61K 47/32 (2006.01) A61K 47/24 (2006.01) A61K 47/10 (2006.01) (21) Application number: 06779420.6 (86) International application number: (22) Date of filing: 14.09.2006 PCT/GB2006/003408 (87) International publication number: WO 2007/031753 (22.03.2007 Gazette 2007/12) (54) Monophasic film-forming composition for topical administration Monophasische filmbildende Zusammensetzung zum topischen Auftrag Composition monophase formant un film pour l’administration à voie topique (84) Designated Contracting States: • JONES, Stuart, Allen AT BE BG CH CY CZ DE DK EE ES FI FR GB GR London SE3 0XA (GB) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR (74) Representative: Lord, Hilton David Marks & Clerk LLP (30) Priority: 14.09.2005 GB 0518769 90 Long Acre London (43) Date of publication of application: WC2E 9RA (GB) 18.06.2008 Bulletin 2008/25 (56) References cited: (73) Proprietor: Medpharm Limited WO-A2-01/43722 US-A- 4 752 466 Charlbury, US-A- 4 863 721 US-A1- 2004 184 994 Oxfordshire OX7 3RR (GB) US-A1- 2004 213 744 (72) Inventors: • BROWN, Marc, Barry Hertfordshire WD19 4QQ (GB) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Topical Therapy As a Treatmentfor Brachioradial
Journal of Case Reports: Open Access Case report Open Access Topical Therapy as a Treatmentfor Brachioradial Pruritis: a Case Report Brianna De Souza M.D, Amy McMichael M.D* Department of Dermatology, Wake Forest University School of Medicine, Winston-Salem, North Carolina *Corresponding author: Amy McMichael, MD Department of Dermatology, Wake Forest Baptist Medical Center,1 Medical Center Blvd, Winston-Salem, NC 27157, Phone: 336-716-7882, Email: [email protected] Received Date: April 12, 2019 Accepted Date: May 06, 2019 Published Date: May 08, 2019 Citation: Brianna De Souza (2019) Topical Therapy as a Treatmentfor Brachioradial Pruritis: a Case Report. Case Reports: Open Access 4: 1-5. Abstract Management of brachioradial pruritus (BRP) presents a formidable challenge to dermatologists and neurologists. BRP is a rare, neurocutaneous condition characterized by sharply localized, chronic pain with associated itching, burning, stinging, and or tingling sensation. Effective care of this patient population is confounded by limitations within the litera- ture, comprised of case series and case reports. We present a case of one middle-aged female with a chronic history of BRP recalcitrant to the following oral therapies: pregabalin, gabapentin, mirtazapine, prednisone, and amitriptyline, as well as topical triamcinolone. After being evaluated in the clinic, the patient was started on combination therapy withKetamine 10%, Amitriptyline 5%, and Lidocaine 5% topical cream to which she responded. Keywords: Brachioradial pruritus, Brachioradial, Pruritus, Neurocutaneous ©2019 The Authors. Published by the JScholar under the terms of the Crea- tive Commons Attribution License http://creativecommons.org/licenses/ by/3.0/, which permits unrestricted use, provided the original author and source are credited. -

F 009 035 Benzocaine 20%, Lidocaine Hydrochoride 7
MEDISCA® NETWORK INC. TECHNICAL SUPPORT SERVICES FORMULATION CHEMISTRY DEPARTMENT TOLL-FREE: 866-333-7811 TELEPHONE: 514-905-5096 FAX: 514-905-5097 [email protected] 1/4/2021; Page 1 Suggested Benzocaine 20%, Lidocaine Hydrochloride 7%, Tetracaine Hydrochloride 7% FIN F 009 035 Formula Topical Gel (Suspension, 30 g) SUGGESTED FORMULATION Lot Expiry Ingredient Listing Qty. Unit NDC # Supplier Number Date Benzocaine, USP 6.000 g Lidocaine Hydrochloride, USP TBD Tetracaine Hydrochloride, USP 2.100 g Polysorbate 80, NF 0.5 mL Ethoxy Diglycol, NF 0.5 mL Medisca VersaPro™ Anhydrous Base 1.50 g Medisca VersaPro™ Anhydrous Base TBD MEDISCA® NETWORK INC. TECHNICAL SUPPORT SERVICES FORMULATION CHEMISTRY DEPARTMENT TOLL-FREE: 866-333-7811 TELEPHONE: 514-905-5096 FAX: 514-905-5097 [email protected] 1/4/2021; Page 2 Suggested Benzocaine 20%, Lidocaine Hydrochloride 7%, Tetracaine Hydrochloride 7% FIN F 009 035 Formula Topical Gel (Suspension, 30 g) SPECIAL PREPARATORY CONSIDERATIONS Ingredient-Specific Information Benzocaine, Tetracaine Hydrochloride, Polysorbate Light Sensitive (protect from light whenever possible): 80 Tetracaine Hydrochloride, Polysorbate 80, Ethoxy Hygroscopic (protect from moisture whenever possible): Diglycol Oxygen Sensitive (protect from air whenever possible): Polysorbate 80 Narrow Therapeutic Index Lidocaine Hydrochloride Suggested Preparatory Guidelines ■ Non-Sterile Preparation □ Sterile Preparation Processing Error / To account for processing error considerations during preparation, it is suggested to Testing Considerations: measure an additional 12 to 15% of the required quantities of ingredients. Special Instruction: This formula may contain one or more Active Pharmaceutical Ingredients (APIs) that may be classified as hazardous, please refer & verify the current NIOSH list of Antineoplastic and Other Hazardous Drugs in Healthcare Settings. -

Meta-Xylene: Identification of a New Antigenic Entity in Hypersensitivity
Clinical Communications Meta-xylene: identification of a new Our patient is a 53-year-old male who presented in 2003 with antigenic entity in hypersensitivity contact dermatitis after the use of lidocaine and disinfection with reactions to local anesthetics chlorhexidine. In 2006, an extensive skin testing by patch, prick, Kuntheavy Ing Lorenzini, PhDa, intradermal sampling, and graded challenge was performed as b c followed. The patch tests were performed with the thin layer Fabienne Gay-Crosier Chabry, MD , Claude Piguet, PhD , rapid use epicutaneous test, using the caine mix (benzocaine, a and Jules Desmeules, MD tetracaine, and cinchocaine), the paraben mix (methyl, ethyl, propyl, butyl, and benzylparaben), and the Balsam of Peru. The Clinical Implications caine and paraben mix were positive, and the patch test was possibly positive for Balsam of Peru. The prick test performed The authors report a patient who presented delayed with preservative-free lidocaine 20 mg/mL (Lidocaïne HCl hypersensitivity reactions to several local anesthetics, all “Bichsel” 2%, Grosse Apotheke Dr. G. Bichsel, Switzerland) was containing a meta-xylene entity, but not to articaine, negative. The intradermal test, performed with lidocaine 20 mg/ which is a thiophene derivative. Meta-xylene could be the mL containing methylparaben (Xylocain 2%, AstraZeneca, immunogenic epitope. Switzerland) with 1/10 and 1/100 dilutions, was negative. The subcutaneous challenge test was performed with 10 mg of preservative-free lidocaine 20 mg/mL (Lidocaïne HCl “Bichsel” TO THE EDITOR: 2%, Grosse Apotheke Dr. G. Bichsel) and lidocaine 20 mg/mL containing methylparaben (Lidocaïne Streuli 2%, Streuli, Hypersensitivity reactions to local anesthetics (LAs) are rare and Switzerland), both of which were positive and had the appear- represent less than 1% of all adverse reactions to LAs. -

Local Anesthetics
Local Anesthetics Introduction and History Cocaine is a naturally occurring compound indigenous to the Andes Mountains, West Indies, and Java. It was the first anesthetic to be discovered and is the only naturally occurring local anesthetic; all others are synthetically derived. Cocaine was introduced into Europe in the 1800s following its isolation from coca beans. Sigmund Freud, the noted Austrian psychoanalyst, used cocaine on his patients and became addicted through self-experimentation. In the latter half of the 1800s, interest in the drug became widespread, and many of cocaine's pharmacologic actions and adverse effects were elucidated during this time. In the 1880s, Koller introduced cocaine to the field of ophthalmology, and Hall introduced it to dentistry Overwiev Local anesthetics (LAs) are drugs that block the sensation of pain in the region where they are administered. LAs act by reversibly blocking the sodium channels of nerve fibers, thereby inhibiting the conduction of nerve impulses. Nerve fibers which carry pain sensation have the smallest diameter and are the first to be blocked by LAs. Loss of motor function and sensation of touch and pressure follow, depending on the duration of action and dose of the LA used. LAs can be infiltrated into skin/subcutaneous tissues to achieve local anesthesia or into the epidural/subarachnoid space to achieve regional anesthesia (e.g., spinal anesthesia, epidural anesthesia, etc.). Some LAs (lidocaine, prilocaine, tetracaine) are effective on topical application and are used before minor invasive procedures (venipuncture, bladder catheterization, endoscopy/laryngoscopy). LAs are divided into two groups based on their chemical structure. The amide group (lidocaine, prilocaine, mepivacaine, etc.) is safer and, hence, more commonly used in clinical practice. -

Antagonism of Lidocaine Inhibition by Open-Channel Blockers That Generate Resurgent Na Current
4976 • The Journal of Neuroscience, March 13, 2013 • 33(11):4976–4987 Cellular/Molecular Antagonism of Lidocaine Inhibition by Open-Channel Blockers That Generate Resurgent Na Current Jason S. Bant,1,3 Teresa K. Aman,2,3 and Indira M. Raman1,2,3 1Interdepartmental Biological Sciences Program, 2Northwestern University Interdepartmental Neuroscience Program, and 3Department of Neurobiology, Northwestern University, Evanston, Illinois 60208 Na channels that generate resurgent current express an intracellular endogenous open-channel blocking protein, whose rapid binding upon depolarization and unbinding upon repolarization minimizes fast and slow inactivation. Na channels also bind exogenous com- pounds, such as lidocaine, which functionally stabilize inactivation. Like the endogenous blocking protein, these use-dependent inhibi- tors bind most effectively at depolarized potentials, raising the question of how lidocaine-like compounds affect neurons with resurgent Na current. We therefore recorded lidocaine inhibition of voltage-clamped, tetrodotoxin-sensitive Na currents in mouse Purkinje neu- rons, which express a native blocking protein, and in mouse hippocampal CA3 pyramidal neurons with and without a peptide from the   cytoplasmic tail of NaV 4 (the 4 peptide), which mimics endogenous open-channel block. To control channel states during drug exposure, lidocaine was applied with rapid-solution exchange techniques during steps to specific voltages. Inhibition of Na currents by lidocaine was diminished by either the 4 peptide or the native blocking protein. In peptide-free CA3 cells, prolonging channel opening with a site-3 toxin, anemone toxin II, reduced lidocaine inhibition; this effect was largely occluded by open-channel blockers, suggesting that lidocaine binding is favored by inactivation but prevented by open-channel block. -
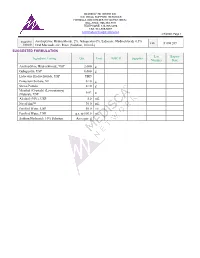
Amitriptyline Hydrochloride 2%, Gabapentin 6%, Lidocaine Hydrochloride 0.5% FIN F 008 269 Formula Oral Mucoadhesive Rinse (Solution, 100 Ml)
MEDISCA® NETWORK INC. TECHNICAL SUPPORT SERVICES FORMULATION CHEMISTRY DEPARTMENT TOLL-FREE: 866-333-7811 TELEPHONE: 514-905-5096 FAX: 514-905-5097 [email protected] 4/7/2020; Page 1 Suggested Amitriptyline Hydrochloride 2%, Gabapentin 6%, Lidocaine Hydrochloride 0.5% FIN F 008 269 Formula Oral Mucoadhesive Rinse (Solution, 100 mL) SUGGESTED FORMULATION Lot Expiry Ingredient Listing Qty. Unit NDC # Supplier Number Date Amitriptyline Hydrochloride, USP 2.000 g Gabapentin, USP 6.000 g Lidocaine Hydrochloride, USP TBD Potassium Sorbate, NF 0.10 g Stevia Powder 0.10 g Menthol (Crystals) (Levorotatory) 0.02 g (Natural), USP Alcohol (95%), USP 5.0 mL NovaFilm™ 30.0 mL Purified Water, USP 50.0 mL Purified Water, USP q.s. to 100.0 mL Sodium Hydroxide 10% Solution As required MEDISCA® NETWORK INC. TECHNICAL SUPPORT SERVICES FORMULATION CHEMISTRY DEPARTMENT TOLL-FREE: 866-333-7811 TELEPHONE: 514-905-5096 FAX: 514-905-5097 [email protected] 4/7/2020; Page 2 Suggested Amitriptyline Hydrochloride 2%, Gabapentin 6%, Lidocaine Hydrochloride 0.5% FIN F 008 269 Formula Oral Mucoadhesive Rinse (Solution, 100 mL) SPECIAL PREPARATORY CONSIDERATIONS Ingredient-Specific Information Light Sensitive (protect from light whenever possible): Amitriptyline Hydrochloride, Gabapentin Hygroscopic (protect from moisture whenever possible): Stevia Powder Narrow Therapeutic Index Lidocaine Hydrochloride Suggested Preparatory Guidelines ■ Non-Sterile Preparation □ Sterile Preparation Processing Error / To account for processing error and pH testing considerations during preparation, it is Testing Considerations: suggested to measure an additional 3 to 5% of the required quantities of ingredients. Special Instruction: This formula may contain one or more Active Pharmaceutical Ingredients (APIs) that may be classified as hazardous, please refer & verify the current NIOSH list of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016. -

The Effects of Lidocaine and Mefenamic Acid on Post-Episiotomy
Shiraz E-Med J. 2016 March; 17(3):e36286. doi: 10.17795/semj36286. Published online 2016 March 27. Research Article The Effects of Lidocaine and Mefenamic Acid on Post-Episiotomy Pain: A Comparative Study Masoumeh Delaram,1,* Lobat Jafar Zadeh,2 and Sahand Shams3 1Faculty of Nursing and Midwifery, Shahrekord University of Medical Sciences, Shahrekord, IR Iran 2Faculty of Medicine, Shahrekord University of Medical Sciences, Shahrekord, IR Iran 3Faculty of Veterinary Medicine, Shahrekord University, Shahrekord, IR Iran *Corresponding author: Masoumeh Delaram, Faculty of Nursing and Midwifery, Shahrekord University of Medical Sciences, Shahrekord, IR Iran. Tel: +98-3813335648, Fax: +98-3813346714, E-mail: [email protected] Received 2016 January 13; Revised 2016 February 29; Accepted 2016 March 04. Abstract Background: Most women suffer pain following an episiotomy and oral non-steroidal anti-inflammatory drugs are commonly used for pain relief. Due to the gastrointestinal side effects of oral drugs, it seems that women are more accepting of topical medications for pain relief. Objectives: Therefore, the aim of this study was to compare the effects of lidocaine and mefenamic acid on post-episiotomy pain. Patients and Methods: This clinical trial was carried out in 2011. It involved sixty women with singleton pregnancy who were given an episiotomy at 38 to 42 weeks of gestation. The participants were randomly divided into two groups. One group received 2% lido- caine cream (n = 30), while the other group received 250 mg of mefenamic acid (n = 30). The data were collected via a questionnaire and a visual analog scale. Pain intensity was compared from the first complaint by the mother and at 6, 12, and 24 hours after the delivery in both groups. -

Adverse Effects of Medications on Oral Health
Adverse Effects of Medications on Oral Health Dr. James Krebs, BS Pharm, MS, PharmD Director of Experiential Education College of Pharmacy, University of New England Presented by: Rachel Foster PharmD Candidate, Class of 2014 University of New England October 2013 Objectives • Describe the pathophysiology of various medication-related oral reactions • Recognize the signs and symptoms associated with medication-related oral reactions • Identify the populations associated with various offending agents • Compare the treatment options for medication-related oral reactions Medication-related Oral Reactions • Stomatitis • Oral Candidiasis • Burning mouth • Gingival hyperplasia syndrome • Alterations in • Glossitis salivation • Erythema • Alterations in taste Multiforme • Halitosis • Oral pigmentation • Angioedema • Tooth discoloration • Black hairy tongue Medication-related Stomatitis • Clinical presentation – Aphthous-like ulcers, mucositis, fixed-drug eruption, lichen planus1,2 – Open sores in the mouth • Tongue, gum line, buccal membrane – Patient complaint of soreness or burning http://www.virtualmedicalcentre.com/diseases/oral-mucositis-om/92 0 http://www.virtualmedicalcentre.com/diseases/oral-mucositis-om/920 Medication-related Stomatitis • Offending agents1,2 Medication Indication Patient Population Aspirin •Heart health • >18 years old •Pain reliever • Cardiac patients NSAIDs (i.e. Ibuprofen, •Headache General population naproxen) •Pain reliever •Fever reducer Chemotherapy (i.e. •Breast cancer •Oncology patients methotrexate, 5FU, •Colon