AKPAH EDUKU.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Micro-CT to Document the Coffee Bean Weevil, Araecerus Fasciculatus (Coleoptera: Anthribidae), Inside Field-Collected Coffee Berries (Coffea Canephora)
insects Communication Micro-CT to Document the Coffee Bean Weevil, Araecerus fasciculatus (Coleoptera: Anthribidae), Inside Field-Collected Coffee Berries (Coffea canephora) Ignacio Alba-Alejandre 1 ID , Javier Alba-Tercedor 1 ID and Fernando E. Vega 2,* ID 1 Department of Zoology, Faculty of Sciences, University of Granada, Campus de Fuentenueva, 18071 Granada, Spain; [email protected] (I.A.-A.); [email protected] (J.A.-T.) 2 Sustainable Perennial Crops Laboratory, United States Department of Agriculture, Agricultural Research Service, Beltsville, MD 20705, USA * Correspondence: [email protected]; Tel.: +1-301-504-5101 Received: 22 June 2018; Accepted: 10 August 2018; Published: 14 August 2018 Abstract: The coffee bean weevil, Araecerus fasciculatus (De Geer) (Coleoptera: Anthribidae), is a cosmopolitan insect with >100 hosts, and has been reported as a pest of stored coffee. During a study involving the coffee berry borer, we observed coffee bean weevils emerging from field-collected coffee berries and used micro-computerized tomography (micro-CT) scans to observe the insect inside the berry. Two eggs had eclosed inside the berry, resulting in observations of a newly eclosed adult beetle and a 5th instar larva, each feeding on one of the two seeds. This is the first time since 1775, when the insect was first described, that the insect has been observed inside a coffee berry. Keywords: coffee quality; insect biology; losses; stored coffee; stored product pest 1. Introduction The genus Araecerus Schönherr comprises ca. 75 species [1], with the coffee bean weevil, Araecerus fasciculatus (De Geer) (Coleoptera: Anthribidae), being the most economically important. Chittenden [2,3] coined the name coffee bean weevil and Valentine [1] has published a succinct account on the controversy involving the many different scientific names used for the insect. -

Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas
Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas Neal L. Evenhuis, Lucius G. Eldredge, Keith T. Arakaki, Darcy Oishi, Janis N. Garcia & William P. Haines Pacific Biological Survey, Bishop Museum, Honolulu, Hawaii 96817 Final Report November 2010 Prepared for: U.S. Fish and Wildlife Service, Pacific Islands Fish & Wildlife Office Honolulu, Hawaii Evenhuis et al. — Pagan Island Arthropod Survey 2 BISHOP MUSEUM The State Museum of Natural and Cultural History 1525 Bernice Street Honolulu, Hawai’i 96817–2704, USA Copyright© 2010 Bishop Museum All Rights Reserved Printed in the United States of America Contribution No. 2010-015 to the Pacific Biological Survey Evenhuis et al. — Pagan Island Arthropod Survey 3 TABLE OF CONTENTS Executive Summary ......................................................................................................... 5 Background ..................................................................................................................... 7 General History .............................................................................................................. 10 Previous Expeditions to Pagan Surveying Terrestrial Arthropods ................................ 12 Current Survey and List of Collecting Sites .................................................................. 18 Sampling Methods ......................................................................................................... 25 Survey Results .............................................................................................................. -

Surveying for Terrestrial Arthropods (Insects and Relatives) Occurring Within the Kahului Airport Environs, Maui, Hawai‘I: Synthesis Report
Surveying for Terrestrial Arthropods (Insects and Relatives) Occurring within the Kahului Airport Environs, Maui, Hawai‘i: Synthesis Report Prepared by Francis G. Howarth, David J. Preston, and Richard Pyle Honolulu, Hawaii January 2012 Surveying for Terrestrial Arthropods (Insects and Relatives) Occurring within the Kahului Airport Environs, Maui, Hawai‘i: Synthesis Report Francis G. Howarth, David J. Preston, and Richard Pyle Hawaii Biological Survey Bishop Museum Honolulu, Hawai‘i 96817 USA Prepared for EKNA Services Inc. 615 Pi‘ikoi Street, Suite 300 Honolulu, Hawai‘i 96814 and State of Hawaii, Department of Transportation, Airports Division Bishop Museum Technical Report 58 Honolulu, Hawaii January 2012 Bishop Museum Press 1525 Bernice Street Honolulu, Hawai‘i Copyright 2012 Bishop Museum All Rights Reserved Printed in the United States of America ISSN 1085-455X Contribution No. 2012 001 to the Hawaii Biological Survey COVER Adult male Hawaiian long-horned wood-borer, Plagithmysus kahului, on its host plant Chenopodium oahuense. This species is endemic to lowland Maui and was discovered during the arthropod surveys. Photograph by Forest and Kim Starr, Makawao, Maui. Used with permission. Hawaii Biological Report on Monitoring Arthropods within Kahului Airport Environs, Synthesis TABLE OF CONTENTS Table of Contents …………….......................................................……………...........……………..…..….i. Executive Summary …….....................................................…………………...........……………..…..….1 Introduction ..................................................................………………………...........……………..…..….4 -

Eco-Climatic Assessment of the Potential Establishment of Exotic Insects in New Zealand
Eco-climatic assessment of the potential establishment of exotic insects in New Zealand A thesis submitted in partial fulfillment of the requirements for the Degree of Doctor of Philosophy at Lincoln University by Lora Peacock Lincoln University 2005 Contents Abstract of a thesis submitted in partial fulfillment of the requirements for the Degree of PhD Eco-climatic assessment of the potential establishment of exotic insects in New Zealand Lora Peacock To refine our knowledge and to adequately test hypotheses concerning theoretical and applied aspects of invasion biology, successful and unsuccessful invaders should be compared. This study investigated insect establishment patterns by comparing the climatic preferences and biological attributes of two groups of polyphagous insect species that are constantly intercepted at New Zealand's border. One group of species is established in New Zealand (n = 15), the other group comprised species that are not established (n = 21). In the present study the two groups were considered to represent successful and unsuccessful invaders. To provide background for interpretation of results of the comparative analysis, global areas that are climatically analogous to sites in New Zealand were identified by an eco climatic assessment model, CLIMEX, to determine possible sources of insect pest invasion. It was found that south east Australia is one of the regions that are climatically very similar to New Zealand. Furthermore, New Zealand shares 90% of its insect pest species with that region. South east Australia has close trade and tourism links with New Zealand and because of its proximity a new incursion in that analogous climate should alert biosecurity authorities in New Zealand. -
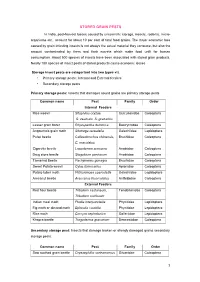
Stored Grain Pests
STORED GRAIN PESTS In India, post-harvest losses caused by unscientific storage, insects, rodents, micro- organisms etc., account for about 10 per cent of total food grains. The major economic loss caused by grain infesting insects is not always the actual material they consume, but also the amount contaminated by them and their excreta which make food unfit for human consumption. About 500 species of insects have been associated with stored grain products. Nearly 100 species of insect pests of stored products cause economic losses Storage insect pests are categorized into two types viz. • Primary storage pests : Internal and External feeders • Secondary storage pests Primary storage pests: Insects that damages sound grains are primary storage pests Common name Pest Family Order Internal Feeders Rice weevil Sitophilus oryzae, Curculionidae Coleoptera S. zeamais, S. granarius Lesser grain borer Rhyzopertha dominica Bostrychidae Coleoptera Angoumois grain moth Sitotroga cerealella Gelechiidae Lepidoptera Pulse beetle Callosobruchus chinensis, Bruchidae Coleoptera C. maculatus Cigarette beetle Lasioderma sericorne Anobiidae Coleoptera Drug store beetle Stegobium paniceum Anobiidae Coleoptera Tamarind Beetle Pachymeres gonagra Bruchidae Coleoptera Sweet Potato weevil Cylas formicarius Apionidae Coleoptera Potato tuber moth Phthorimoea operculella Gelechiidae Lepidoptera Arecanut beetle Araecerus fasciculatus Anthribidae Coleoptera External Feeders Red flour beetle Tribolium castaneum, Tenebrionidae Coleoptera Tribolium confusum Indian meal -

Die Rüsselkäfer (Coleoptera, Curculionoidea) Der Schweiz – Checkliste Mit Verbreitungsangaben Nach Biogeografischen Regionen
MITTEILUNGEN DER SCHWEIZERISCHEN ENTOMOLOGISCHEN GESELLSCHAFT BULLETIN DE LA SOCIÉTÉ ENTOMOLOGIQUE SUISSE 83: 41–118, 2010 Die Rüsselkäfer (Coleoptera, Curculionoidea) der Schweiz – Checkliste mit Verbreitungsangaben nach biogeografischen Regionen CHRISTOPH GERMANN Natur-Museum Luzern, Kasernenplatz 6, 6003 Luzern und Naturhistorisches Museum der Burger ge - meinde Bern, Bernastrasse 15, 3006 Bern; Email: [email protected] The weevils of Switzerland – Checklist (Coleoptera, Curculionoidea), with distribution data by bio - geo graphic regions. – A checklist of the Swiss weevils (Curculionoidea) including distributional pat- terns based on 6 bio-geographical regions is presented. Altogether, the 1060 species and subspecies out of the 10 families are composed of 21 Anthribidae, 129 Apionidae, 3 Attelabidae, 847 Cur cu lio - ni dae, 7 Dryophthoridae, 9 Erirhinidae, 13 Nanophyidae, 3 Nemonychidae, 3 Raymondionymidae, and 25 Rhynchitidae. Further, 13 synanthropic, 42 introduced species as well as 127 species, solely known based on old records, are given. For all species their synonymous names used in Swiss litera- ture are provided. 151 species classified as doubtful for the Swiss fauna are listed separately. Keywords: Curculionoidea, Checklist, Switzerland, faunistics, distribution EINLEITUNG Rüsselkäfer im weiteren Sinn (Curculionoidea) stellen mit über 62.000 bisher beschriebenen Arten und gut weiteren 150.000 zu erwartenden Arten (Oberprieler et al. 2007) die artenreichste Käferfamilie weltweit dar. Die ungeheure Vielfalt an Arten, Farben und Formen oder verschiedenartigsten Lebensweisen und damit ein - her gehenden Anpassungen fasziniert immer wieder aufs Neue. Eine auffällige Gemein samkeit aller Rüsselkäfer ist der verlängerte Kopf, welcher als «Rostrum» (Rüssel) bezeichnet wird. Rüsselkäfer sind als Phyto phage stets auf ihre Wirts- pflanzen angewiesen und folgen diesen bei uns von Unter wasser-Biotopen im pla- naren Bereich (u.a. -

EU Project Number 613678
EU project number 613678 Strategies to develop effective, innovative and practical approaches to protect major European fruit crops from pests and pathogens Work package 1. Pathways of introduction of fruit pests and pathogens Deliverable 1.3. PART 7 - REPORT on Oranges and Mandarins – Fruit pathway and Alert List Partners involved: EPPO (Grousset F, Petter F, Suffert M) and JKI (Steffen K, Wilstermann A, Schrader G). This document should be cited as ‘Grousset F, Wistermann A, Steffen K, Petter F, Schrader G, Suffert M (2016) DROPSA Deliverable 1.3 Report for Oranges and Mandarins – Fruit pathway and Alert List’. An Excel file containing supporting information is available at https://upload.eppo.int/download/112o3f5b0c014 DROPSA is funded by the European Union’s Seventh Framework Programme for research, technological development and demonstration (grant agreement no. 613678). www.dropsaproject.eu [email protected] DROPSA DELIVERABLE REPORT on ORANGES AND MANDARINS – Fruit pathway and Alert List 1. Introduction ............................................................................................................................................... 2 1.1 Background on oranges and mandarins ..................................................................................................... 2 1.2 Data on production and trade of orange and mandarin fruit ........................................................................ 5 1.3 Characteristics of the pathway ‘orange and mandarin fruit’ ....................................................................... -

The Ecology of Insect Pest Populations in Maize
THE ECOLOGY OF INSECT PEST POPULATIONS IN MAIZE STORAGE CRIBS IN NIGERIA by Richard Hugh Markham, B.A. (Nat. Sci.) A thesis submitted for the degree of Doctor of Philosophy of the University of London and the Diploma of Imperial College. Tropical Stored Products Centre, (Overseas Development Administration), London Road, Slough, Berkshire. March 1981 THE ECOLOGY OF INSECT PEST POPULATIONS IN MAIZE STORAGE CRIBS IN NIGERIA Richard Hugh Markham Abstract This study considered the insect populations infesting white dent maize stored in well-ventilated cribs at two localities in South West Nigeria. The pest complex was dominated by Sitophilus zeamais (Col.: Curculionidae) but included a great diversity of other pest species and natural enemies. The incidence of individual species was studied from pre-harvest infestation through six to ten months of storage and was shown to follow a consistent succession. The spatial distribution of insects within a crib was not uniform and individual species showed consistent patterns of distribution at a particular time. The seasonal incidence and distribution patterns of major species are discussed in terms of observed changes in grain moisture content, temperature and grain damage. The roles of intra- and interspecific relationships in limiting populations are considered. Sitophilus populations rapidly reach a 'plateau' and it is concluded that further significant increase is prevented by this insect's responses to its own high population density. The relationship between the field infestation and subsequent pest population increase in store is considered with particular reference to the effects of time of harvest, removal or retention of the husks and of damage* caused in the field by Lepidoptera larvae. -

Beiträge Zum Göttinger Umwelthistorischen Kolloquium 2008
Seit seiner Gründung vor annähernd 25 Jahren hat sich das Göttinger Umwelthis- torische Kolloquium zu einer Einrichtung entwickelt, welche die vielfältigen, Graduiertenkolleg Interdisziplinäre Umweltgeschichte thematisch einschlägigen Aktivitäten des Standortes wie auch des deutschspra- chigen Raumes durch Austausch von Forschungsergebnissen und Sichtweisen bündelt. Von hier haben auch einige Unternehmungen ihren Ausgang genom- men, welche zum heutigen Profil der Umweltgeschichte spürbar beitrugen. Bernd Herrmann (Hg.) Der Band vereinigt Beiträge zum Kolloquium des Sommersemesters 2008 und des Wintersemesters 2008/09. Beiträge zum Göttinger Umwelthistorischen Kolloquium 2008 - 2009 Bernd Herrmann (Hg.) Beiträge zum Göttinger Umwelthistorischen Kolloquium 2008-2009 ISBN: 978-3-940344-97-7 Universitätsverlag Göttingen Universitätsverlag Göttingen Bernd Herrmann (Hg.) Beiträge zum Göttinger Umwelthistorischen Kolloquium 2008 – 2009 This work is licensed under the Creative Commons License 2.0 “by-nc-nd”, allowing you to download, distribute and print the document in a few copies for private or educational use, given that the document stays unchanged and the creator is mentioned. Commercial use is not covered by the licence. erschienen im Universitätsverlag Göttingen 2009 Bernd Herrmann (Hg.) Beiträge zum Göttinger Umwelthistorischen Kolloquium 2008 - 2009 Graduiertenkolleg Interdisziplinäre Umweltgeschichte Universitätsverlag Göttingen 2009 Bibliographische Information der Deutschen Nationalbibliothek Die Deutsche Nationalbibliothek verzeichnet -

2018 Myanmar PERSUAP
BUREAUS FOR ASIA AND FOOD SECURITY PESTICIDE EVALUATION REPORT AND SAFE USE ACTION PLAN (PERSUAP) IEE AMENDMENT: §216.3(B) PESTICIDE PROCEDURES PROJECT/ACTIVITY DATA Project/Activity Name: Feed the Future Cambodia Harvest II (“Harvest II”) Amendment #: 1 Geographic Location: South East Asia (Cambodia) Implementation Start/End: 1/1/2017 to 12/31/2021 Implementing Partner(s): Abt Associates, International Development Enterprises (iDE), Emerging Markets Consulting (EMC) Tracking ID/link: Tracking ID/link of Related IEE: Asia 16-042 https://ecd.usaid.gov/repository/pdf/46486.pdf Tracking ID/link of Other Related Analyses: ORGANIZATIONAL/ADMINISTRATIVE DATA Implementing Operating Unit: Cambodia Funding Operating Unit: Bureau for Asia Initial Funding Account(s): $17.5 M Total Funding Amount: Amendment Funding Date / Amount: April 2017-April 2022 Other Affected Units: Bureau for Food Security Lead BEO Bureau: Asia Prepared by: Alan Schroeder, PhD, MBA; Kim Hian Seng, PhD, with support from Abt Associates and iDE Date Prepared: 8/2/2018 ENVIRONMENTAL COMPLIANCE REVIEW DATA Analysis Type: §216.3(B) Pesticide Procedures - PERSUAP Environmental Determination: Negative Determination with Conditions PERSUAP Procedures Expiration Date: 12/31/2021 Climate Risks Considerations / Conditions 2018 Feed the Future Cambodia Harvest II PERSUAP 1 ACRONYMS AI Active Ingredient A/COR Agreement/Contracting Officer’s Representative ASIA Bureau for Asia BEO Bureau Environmental Officer BMP Best Management Practice BRC British Retail Consortium BT Bacillus thuringiensis -

Proceedings of the United States National Museum
STUDIES OF THE NORTH AMERICAN WEEVILS BELONG- ING TO THE SUPERFAMILY PLATYSTOMOIDEA By W. DwiGHT Pierce Formerly Entomologist, Southern Field Crop Insect Investigations, Bureau of Entomology, United States Department of Agriculture The superfamily Platystomoidea Pierce (1916) is composed of those weevils classed by LeConte and Horn and other authors under the family Anthribidae. In planning a more comprehensive classi- fication of the Rhynchophora it has been found best to raise the old conceptions of families to a superfamily rank. The oldest valid name in the superfamily is Platystomos (Hell- wig) Schneider (1791), and hence it gives its name to the family in which it is to be placed and also to the superfamily. The group is composed of individuals with clavate, nongeniculate antennae, flexible maxillary palpi, a distinct labrum, globose anterior coxae, and an exposed pygidium. TABLE OF FAMILIES OF PLATYSTOMOIDEA 1, Prothorax with transverse carina near base; third joint of tarsus usually largely inclosed in the second 2. Prothorax without transverse carina near base; third joint of tarsus free from second Bruehelidae Pierce (1916) (not North American). 2. Antennae inserted on the sides of the rostrum ; labial palpi three-jointed (Pleurocera) Platystomidae Pierce (1916). Antennae inserted on the upper surface of the rostrum near the eyes; labial palpi at least sometimes four-jointed (Anocera), Choragidae Des Gozis (1882). The transverse carina of the prothorax is of definite systematic value, as it represents the suture between two distinct portions of the thorax. This will be evident by examining the series of illustrations herewith presented. The area in front of this carina and laterally inclosed by it when the carina turns forward at the sides is the scutellum. -

Diversity and Functions of Yeast Communities Associated with Insects
microorganisms Review Diversity and Functions of Yeast Communities Associated with Insects Simon Malassigné, Guillaume Minard, Laurent Vallon, Edwige Martin, Claire Valiente Moro and Patricia Luis * Univ Lyon, Université Claude Bernard Lyon 1, CNRS, INRAE, VetAgro Sup, UMR Ecologie Microbienne, F-69622 Villeurbanne, France; [email protected] (S.M.); [email protected] (G.M.); [email protected] (L.V.); [email protected] (E.M.); [email protected] (C.V.M.) * Correspondence: [email protected] Abstract: Following the concept of the holobiont, insect-microbiota interactions play an important role in insect biology. Many examples of host-associated microorganisms have been reported to drastically influence insect biological processes such as development, physiology, nutrition, survival, immunity, or even vector competence. While a huge number of studies on insect-associated micro- biota have focused on bacteria, other microbial partners including fungi have been comparatively neglected. Yeasts, which establish mostly commensal or symbiotic relationships with their host, can dominate the mycobiota of certain insects. This review presents key advances and progress in the research field highlighting the diversity of yeast communities associated with insects, as well as their impact on insect life-history traits, immunity, and behavior. Keywords: insect-microbiota interactions; mycobiota; yeast communities; insects Citation: Malassigné, S.; Minard, G.; Vallon, L.; Martin, E.; Valiente Moro, C.; Luis, P. Diversity and Functions of Yeast Communities Associated with 1. Introduction Insects. Microorganisms 2021, 9, 1552. With nearly one million described species and 5.5 million estimated ones, insects https://doi.org/10.3390/ represent more than 80% of the animal biodiversity on Earth [1].