Year-Round N2O Production by Benthic Nox Reduction in a Monomictic South-Alpine Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Drives Warming Trends in Streams? a Case Study from the Alpine Foothills
RIVER RESEARCH AND APPLICATIONS River Res. Applic. 31: 663–675 (2015) Published online 8 May 2014 in Wiley Online Library (wileyonlinelibrary.com) DOI: 10.1002/rra.2763 WHAT DRIVES WARMING TRENDS IN STREAMS? A CASE STUDY FROM THE ALPINE FOOTHILLS F. LEPORI*, M. POZZONI AND S. PERA Institute of Earth Sciences, Canobbio, Switzerland ABSTRACT We investigated the effects of climate warming and land-use changes on the temperature and discharge of seven Swiss and Italian streams in the catchment of Lake Lugano. In addition, we attempted to predict future stream conditions based on regional climate scenarios. Between 1976 and 2012, the study streams warmed by 1.5–4.3 °C, whereas discharge showed no long-term trends. Warming trends were driven mainly by catchment urbanization and two large-scale climatic oscillations, the North Atlantic Oscillation and the Atlantic Multidecadal Oscillation. In comparison, independent influences by radiative forcing due to increased atmospheric CO2 were uncertain. However, radiative forcing was predicted to further increase stream temperature (to +3–7 °C), reduce summer discharge (to À46%) and increase winter discharge (to +96%) between the present and 2070–2099. These results provide new insights into the drivers of long-term temperature and discharge trends in European streams subject to multiple impacts. The picture emerging is one of transition, where greenhouse-gas forcing is gaining ground over climate oscillations and urbanization, the drivers of past trends. This shift would impress a more directional nature upon future changes in stream temperature and discharge, and extend anthropogenic warming to rural streams. Diffusing future impacts on stream ecosystems would require adaptation measures at local to national scales and mitigation of greenhouse-gas emissions at the global scale. -

PANNELLO Di CONTROLLO Sullo Stato E Sull'evoluzione Delle Acque Del Lago Di Lugano
PANNELLO di CONTROLLO Sullo stato e sull'evoluzione delle acque del Lago di Lugano Il documento è stato redatto a cura del Segretariato Tecnico della CIPAIS ANNO 2018 Commissione Internazionale per la Protezione delle Acque Italo – Svizzere SOMMARIO Premessa 2 L3 9: Antibiotico resistenza nei batteri lacustri 26 Il Territorio di interesse per la CIPAIS 3 L3 11: Produzione primaria 27 Il Lago di Lugano 4 L3 12: Concentrazione media di fosforo e azoto 28 Indicatori del Pannello di controllo 5 L3 13: Concentrazione dell'ossigeno di fondo 29 Quadro Ambientale del 2017: aspetti limnologici 6 Tematica: Inquinamento delle acque Quadro Ambientale del 2017: sostanze inquinanti 7 L4 1: Carico di fosforo totale e azoto totale in ingresso a lago 30 Comparto: Ambiente lacustre L4 2: Microinquinanti nell’ecosistema lacustre 31, 32 Tematica: Antropizzazione e uso del territorio e delle risorse naturali Comparto: Bacino idrografico L1 1: Prelievo ad uso potabile 8 Tematica: Antropizzazione e uso del territorio e delle risorse naturali L1 2: Zone balneabili 9 B1 1: Uso del suolo 33 L1 4: Pescato 10 B1 2: Percorribilità fluviale da parte delle specie ittiche 34 L1 5: Potenziale di valorizzazione delle rive 11, 12 Tematica: Ecologia e biodiversità Tematica: Idrologia e clima B3 1: Elementi chimico - fisici 35 L2 1: Livello lacustre 13 B3 2: Macroinvertebrati bentonici 36 L2 2: Temperatura media delle acque nello strato 0-20 m e profondo 14 Tematica: Inquinamento delle acque L2 3 Massima profondità di mescolamento 15 B4 2: Stato delle opere di risanamento -

Commissariato Italiano Per La Convenzione Italo-Svizzera Sulla Pesca
SEGRETERIA E RECAPITO CORRISPONDENZA Commissariato italiano COMMISSARIATO ITALIANO PER LA PESCA c/o CNR Istituto di Ricerca Sulle Acque via Tonolli 50 28922 Verbania Pallanza per la Convenzione tel. 0323 518327 fax 0323 55651 posta certificata [email protected] italo-svizzera sulla pesca e-mail segreteria [email protected] Codice Fiscale 93007650034 DATA N. Argomento ordinanze pag. 14/06/21 03/21 Pescate di sfoltimento di agone nel Lago Maggiore ….………….………….………….………….……. 1 03/06/21 02/21 Proroga scadenza dell’ordinanza n. 02/15 ……….………….………….………….………….…………. 2 11/01/21 01/21 Libretto segna pesci della pesca dilettantistica nelle acque lombarde soggette alla CISPP ….…..… 3 23/12/19 03/19 Libretto segna catture della pesca professionale nelle acque lacustri lombarde della CISPP ……… 4 16/12/19 02/19 Divieti di pesca allo sbocco e imbocco del F. Tresa a Lavena Ponte Tresa ………………………….. 5 21/12/18 03/18 Nuovo Regolamento di Applicazione della Convenzione (R.d.A . 2019)………………………………. 6 15/06/16 01/16 Orari della pesca professionale nelle acque italiane del Lago di Lugano………………………………. 7 08/02/16 C1/16 Impiego delle reti volanti nel Lago Maggiore ……………………………………………..………………. 8 10/11/15 14/15 Protezione popolamenti coregoni, lucioperca, persico e trota nelle acque italiane del L.Maggiore … 9 01/01/15 02/15 Protezione della fauna ittica alla foce dei principali tributari dei laghi Maggiore e di Lugano ……….. 10 01/01/15 03/15 Divieto di pesca dell’agone nelle acque italiane del Lago Maggiore ……………………………………. 11 01/01/15 05/15 Orari della pesca con attrezzi professionali nelle acque italiane del Lago Maggiore ......................... -

Piano Zone Biglietti E Abbonamenti 2021
Comunità tariffale Arcobaleno – Piano delle zone arcobaleno.ch – [email protected] per il passo per Geirett/Luzzone per Göschenen - Erstfeld del Lucomagno Predelp Carì per Thusis - Coira per il passo S. Gottardo Altanca Campo (Blenio) S. Bernardino (Paese) Lurengo Osco Campello Quinto Ghirone 251 Airolo Mairengo 243 Pian S. Giacomo Bedretto Fontana Varenzo 241 Olivone Tortengo Calpiogna Mesocco per il passo All’Acqua Piotta Ambrì Tengia 25 della Novena Aquila 245 244 Fiesso Rossura Ponto Soazza Nante Rodi Polmengo Valentino 24 Dangio per Arth-Goldau - Zurigo/Lucerna Fusio Prato Faido 250 (Leventina) 242 Castro 331 33 Piano Chiggiogna Torre Cabbiolo Mogno 240 Augio Rossa S. Carlo di Peccia Dalpe Prugiasco Lostallo 332 Peccia Lottigna Lavorgo 222 Sorte Menzonio Broglio Sornico Sonogno Calonico 23 S. Domenica Prato Leontica Roseto 330 Cama Brontallo 230 Acquarossa 212 Frasco Corzoneso Cauco Foroglio Nivo Giornico Verdabbio Mondada Cavergno 326 Dongio 231 S. Maria Leggia Bignasco Bosco Gurin Gerra (Verz.) Chironico Ludiano Motto (Blenio) 221 322 Sobrio Selma 32 Semione Malvaglia 22 Grono Collinasca Someo Bodio Arvigo Cevio Brione (Verz.) Buseno Personico Pollegio Loderio Cerentino Linescio Riveo Giumaglio Roveredo (GR) Coglio Campo (V.Mag.) 325 Osogna 213 320 Biasca 21 Lodano Lavertezzo 220 Cresciano S. Vittore Cimalmotto 324 Maggia Iragna Moghegno Lodrino Claro 210 Lumino Vergeletto Gresso Aurigeno Gordevio Corippo Vogorno Berzona (Verzasca) Prosito 312 Preonzo 323 31 311 Castione Comologno Russo Berzona Cresmino Avegno Mergoscia Contra Gordemo Gnosca Ponte Locarno Gorduno Spruga Crana Mosogno Loco Brolla Orselina 20 Arbedo Verscio Monti Medoscio Carasso S. Martino Brione Bellinzona Intragna Tegna Gerra Camedo Borgnone Verdasio Minusio s. -

Commissione Internazionale Per La Protezione Delle Acque Italo-Svizzere
ISSN: 1013-8080 Commissione Internazionale per la protezione delle acque italo-svizzere Ricerche sull'evoluzione del Lago di Lugano Aspetti limnologici Programma quinquennale 1998-2002 Campagna 2002 e Rapporto quinquennale 1998-2002 Ufficio Protezione e Depurazione Acque Sezione Protezione Aria, Acqua e Suolo Dipartimento del Territorio - Cantone Ticino I dati riportati nel presente volume possono essere utilizzati purchè se ne citi la fonte come segue: Ufficio Protezione e Depurazione Acque (UPDA), 2003: “Ricerche sull’evoluzione del Lago di Lugano. Aspetti limnologici. Programma quinquennale 1998-2002. Campagna 2002 e rapporto quinquennale 1998-2002.” Commissione Internazionale per la Protezione delle Acque Italo-Svizzere (Ed.); 110 pp. 3 R I A S S U N T O Questo volume presenta i dati limnologici sul Lago di Lugano raccolti dall'Ufficio Protezione e Depurazione Acque (UPDA) del Cantone Ticino durante la campagna 2002, nell’ambito dell’attività di ricerca della Commissione Internazionale per la Protezione delle Acque Italo-Svizzere svolta a partire dal 1978. Trattandosi dell'ultimo rapporto del quinquennio 1998-2002 è stato inoltre presentato e discusso l'andamento limnologico del lago sul lungo periodo. Le informazioni ottenute nel corso del 2002 permettono di aggiornare le serie storiche disponibili per i principali parametri limnologici, e di descrivere le tendenze evolutive del Lago in relazione agli interventi di depurazione sinora realizzati. Durante l’anno è proseguita l'analisi dettagliata dei carichi esterni di fosforo ai due bacini principali, in modo da verificare in quale misura le opere di risanamento contribuiscano al recupero del corpo idrico. La progressiva riduzione delle concentrazioni di fosforo riscontrata nell’ultimo decennio è proseguita nel bacino sud, mentre si è arrestata all'interno dello strato 0-100 m del bacino nord. -
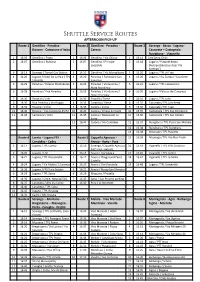
Afternoon Drop-Off 2021-2022
Shuttle Service Routes AFTERNOON PICK-UP Route 1 Gentilino - Paradiso - Route 2 Gentilino - Paradiso - Route 3 Sorengo - Besso - Lugano - Bissone - Campione d’Italia Carona Cassarate – Castagnola - Ruvigliana - Viganello 1 16.05 Gentilino / Posta 1 16.06 Gentilino / Via Chioso 1 16.14 Sant’Anna Clinic 2 16.07 Gentilino / Rubiana 2 16.07 Gentilino / Principe 2 16.18 Lugano / Piazzale Besso Leopoldo (Autopostale) bus stop ‘Via Sorengo’) 3 16.13 Sorengo / Tamoil Gas Station 3 16.10 Gentilino / Via Montalbano 3 16.20 Lugano / TPL Ai Frati 4 16.20 Lugano / Hotel De La Paix / TPL 4 16.20 Paradiso / Funicolare San 4 16.20 Lugano / Via Zurigo / ‘Casaforte’ S.Birgitta Salvatore 5 16.25 Paradiso / Palazzo Mantegazza 5 16.22 Paradiso / Via Guidino / 5 16:22 Lugano / TPL Cappuccine Nizza Residence 6 16.28 Paradiso / Riva Paradiso 6 16.23 Paradiso / Via Guidino / 6 16.30 Lugano / Palazzo dei Congressi Hotel The View 7 16.30 Paradiso / Lido 7 16.30 Pazzallo / Paese 7 16:31 Lugano / TPL Lido 8 16.30 Riva Paradiso / Via Boggia 8 16.33 Carabbia / Paese 8 16.32 Cassarate / TPL Lanchetta 9 16.35 Bissone / Circle 9 16.35 Carona / Ciona 9 16.33 Cassarate / TPL Lago 10 16.40 Bissone / Via Campione 45/55 10 16.40 Carona / Chiesa dei Santi 10 16.35 Castagnola / TPL San Domenico 11 16.43 Campione / Arco 11 16.45 Carona / Restaurant La 11 16.36 Castagnola / TPL San Giorgio Sosta 12 16:47 Carona / Via Colombei 12 16.37 Ruvigliana / TPL Parco San Michele 13 16.38 Ruvigliana / TPL Suvigliana 14 16:38 Albonago / TPL Ruscello Route 4 Loreto - Lugano FFS - Route 5 Cappella -

Arcobaleno. La Scelta Giusta Che Ti Premia
Arcobaleno. La scelta giusta che ti premia. Edizione 2018/19 arcobalenopremia.ch 48 offerte annuali Le offerte dei nostri partner In qualità di abbonati annuali Arcobaleno potete beneficiare in qualsiasi momento dell’anno delle offerte pensate per voi dai nostri partner. Le convenzioni sono suddivise in quattro aree tematiche: Formazione e cultura Shopping e servizi Sport e benessere Viaggi e tempo libero Maggiori informazioni sulle singole offerte sono riportate nelle pagine dedicate ai partner e sul sito arcobalenopremia.ch In aggiunta alle convenzioni quadro, il programma fedeltà propone ogni tre mesi: Offerte stagionali Concorso a premi Le offerte stagionali possono essere visionate sul sito e tramite la nostra newsletter. La newsletter elettronica del programma fedeltà Per essere sempre informati su novità e proposte è sufficiente iscriversi alla newsletter elettronica del programma fedeltà. La newsletter è spedita, di regola, quattro volte all’anno, in corrispondenza del lancio delle offerte stagionali. Visita arcobaleno.ch/newsletter Edizione 2018/19. Con riserva di modifica. I premi e i benefici connessi al programma fedeltà non sono convertibili in denaro. La CTA non è responsabile della qualità delle prestazioni erogate dai partner. Non si tiene corrispondenza in merito ai concorsi promossi in abbinamento al programma fedeltà. Condizioni di utilizzo consultabili su arcobalenopremia.ch. Cartoleria Libreria ABC . Biasca 5% di sconto sui libri e 10% su articoli regalo e ufficio. L’offerta non è cumulabile con altre promozioni particolari. Cinema Teatro . Chiasso Fino al 10% di sconto sugli abbonamenti e fino al 15% sui biglietti. Per beneficiare dello sconto gli abbonati devono presentare l’abbonamento ed il tagliando scaricabile sul sito Arcobaleno. -

Linee Trasporti Pubblici Luganesi SA Valide a Partire Dal 13.12.2020
Linee Trasporti Pubblici Luganesi SA Valide a partire dal 13.12.2020 Giubiasco Bellinzona Origlio- Sureggio- Carnago- Manno- Piano Stampa Locarno Tesserete Tesserete Tesserete Bioggio Capolinea Basilea-Zurigo a Lamone Farer Cadempino Comano Canobbio Stazione Ganna lio Mag te on le P al V Manno di Uovo di Manno 19 Cadempino Municipio Canobbio Cadempino Comano Ronchetto Mercato Resega Studio TV Cureglia Rotonda Comano cio Rotonda c de Resega er Ghia Trevano ta ce V is o Centro Studi P Cr Villa 7 Negroni 19 a ic Resega Term Vezia Paese o Lugano Cornaredo ared i/Corn an i Stadio Est a Ci Cureggia Vi tan Pregassona ren Paese ia B Piazza di Giro Vignola V na ami tr Villa Recreatio Bel Cà Rezzonico a Vi a Viganello za S. Siro lla an sa Seren Vi st Ospedale Vignola Scuole Ca Co Civico Molino Nuovo 2 ovo h i sc ch Scuole a Nu tà ri n z o si F Piazza Molino Nuovo az in er x Ro o Pi l v a sc Praccio Molino Nuovo 2 i ai Mo Un ia M Bo V al al Sole Sassa Via Santa Lucia Zurigo Sacro ta Brè Pianazzo Vicolo Cuore San vecchio a le Paese Paese l Albonago o Autosilo ia edano ag ez ia Balestra Corsolv Ospal Paese ldes Vergiò E It A Genzana - to u o A o a i que Vie l in Si C io Via Balestr Rad Gradinata S. Anton udio Lugano St lao Suvigliana co i Centro o t Scuole Funicolare di ri Ni Cassarate to S. -

Relazione Porlezza Torrenti
GEOPLANET INDICE 1. PREMESSA__________________________________________________________________ 2 1. INQUADRAMENTO GEOGRAFICO _________________________________________ 7 2. INQUADRAMENTO GEOLOGICO __________________________________________ 8 2.1 CENNI PALEOGEOGRAFICI ___________________________________________________ 8 3. COMMENTO ALLA CARTA GEOLOGICO-STRUTTURALE _____________________ 9 3.1 – CARATTERI GEOMORFOLOGICI E GEOLOGICI _________________________________ 9 3.2 – CARATTERI LITOLOGICI _____________________________________________________ 11 3.2.1 Depositi superficiali ___________________________________________________________________ 11 3.2.2 Substrato roccioso ____________________________________________________________________ 13 4. ASPETTI PEDOLOGICI __________________________________________________ 21 5. OSSERVAZIONI CLIMATOLOGICHE ______________________________________ 21 INQUADRAMENTO METEO-CLIMATICO ___________________________________________ 21 5.1.1Temperatura atmosferica ________________________________________________________________ 21 5.1.2 Radiazione solare globale _______________________________________________________________ 22 5.1.3 Precipitazioni _________________________________________________________________________ 23 5.1.4 Intensità dei venti ______________________________________________________________________ 24 6. CARATTERISTICHE METEOROLOGICHE DELL'AREALE LACUSTRE 1998-2007 25 7. REGIME DEL LIVELLO LACUSTRE _______________________________________ 27 7.1 Regime del livello lacustre 1930-1997 _________________________________________________ -

La Via Dei Canti
FORESTA REGIONALE VALSOLDA (CO) LA VIA DEI CANTI Quick link Quick link FORESTA REGIONALE PER SCARICARE VALSOLDA (CO) LE TRACCE SONORE www.ersaf.lombardia.it Barchi Darni 1316 Signora Tecchia a Marda S. Antonio Pianon 1229 n 994 1 Ugino 5 3 0 a Cagon sol 0 Posia 1100 al da Segolone L V Bogno 3 Tegnivo Colla 1610 1071 F Serravada 961 i Rusi di Vora 886 Cavargna d Cureia 1399 Bubegno Monte 1 Colone Zocchetta Cima al 20 0 Burena e Matro l Marsèta Rif. S. Lucio l 1224 Scareglia A. Cottino a Ambesello Corticiasca 1550 Forni Vecchi S. Nazzaro Rugino F V 816 Pianca 1441 Val Cavargna Cardè Lember Maglio Vraghex 1016 S. Lucio Pradè di Colla 1540 S. Giovanni P.te di Lana Gordolina Roncaliva 1000 Piana dellʼUva Carnia Piazza T 9 o r Certara 12 00 r e Albumo 0 00 V n 0 t 4 3 e Cranello 1 Buchi C Neggio Trevin a Ponte Calbino u 80 Insone 12 0 1003 00 c Osnaga l c Roccoli i Piandera o Vora 853 Tavaino Pezza 1379 d Püfin Valpèra Freggio i Carusio C S. Bartolomeo 680 Premestivo Monte a v Val Cavargna 0 880 a Piazzora 0 Roccolo Citella Alpetto Sella C r g F Nagia Curtina 4 n a Piano della 1 Cavada Forcoletta 1334 Pezza Sora 130 0 802 a 0 a 0 Al Ponte Cucco 1148 701 7 Costa Trecciò l Passo della meo Piancaccia La Corte rtolo l v . Ba 870 Cava i S o Paradiso d o C i c c Bertogno l 880 Nendum Alpe Colmine a u a Prato Bello 1484 3 C V Cimadera e 700 00 Grisello t 0 1 n 1 1624 Monte Cucco 2 V 0 e 0 Crisello r rr o 0 T 800 a 0 Seghébbia Monte Piazzola V 3 Dasio 1 582 a l 1354 g Cusino Arla 1100 l Ponte 1 Mugnai l Val Rezzo 0 Dovia 1110 0 Le Spine d Prati della Poma 0 e Costa di Stella Cugnoli i 925 0 n Mattor dei Falchi Corti V 0 V 4 Buggiolo 0 d 1 0 a 9 1300 e 1290 828 i a l Prati di Firovana g g n a Alpe Crisello a i V C S d a i V Madonna dʼArla 1 n l d 3 F 0 i Monti Torre 753 1072 Alpaccio p 0 a 1 20 C 0 i l 947 n a Pramarzio 1587 p B.tta di Usin a 1061 1100 o S. -

Autorita' Ambito Territoriale Ottimale
AUTORITA’ AMBITO TERRITORIALE OTTIMALE Provincia di VARESE INDICE 1 PREMESSA ......................................................................................................................................................................4 2 ACQUISIZIONE DATI IDROGEOLOGICI ED IDROCHIMICI E CRITICITÀ RISCONTRATE .....................6 2.1 QUADRO GENERALE .............................................................................................................................................. 6 2.2 ORGANIZZAZIONE DELLA FASE DI ACQUISIZIONE DATI .......................................................................................... 7 2.3 CRITICITÀ RISCONTRATE ....................................................................................................................................... 7 3 CARATTERI METEOCLIMATICI..............................................................................................................................9 3.1 CENNI DI CLIMATOLOGIA REGIONALE.................................................................................................................... 9 3.2 SUDDIVISIONE METEOCLIMATICA DELLA PROVINCIA DI VARESE ........................................................................ 11 3.3 REGIME PLUVIOMETRICO DELLA PROVINCIA DI VARESE ..................................................................................... 14 3.4 INTERDIPENDENZA PRECIPITAZIONE/FALDA ACQUIFERA ..................................................................................... 22 3.5 MACROCICLI E REVERSIBILITÀ -

0.818.694.54 Accordo Tra Il Governo Svizzero E Il Governo Italiano Concernente La Traslazione Di Salme Nel Traffico Locale Di Confine
Testo originale 0.818.694.54 Accordo tra il Governo svizzero e il Governo italiano concernente la traslazione di salme nel traffico locale di confine Tra la Legazione di Svizzera a Roma e il Ministero italiano degli affari esteri ha avuto luogo in data 11 aprile/14 maggio 1951 uno scambio di note relativo alla traslazione di salme nel traffico locale di confine. La nota svizzera è del seguente tenore: 1. La Convenzione internazionale concernente il trasporto dei cadaveri con- chiusa a Berlino il 10 febbraio 19371 alla quale hanno aderito tanto l’Italia quanto la Svizzera, prevede all’articolo 10 che le sue disposizioni non si ap- plicano al trasporto dei cadaveri nel traffico locale di confine. I due Governi decidono di adottare in proposito la seguente procedura: 2. Il traffico locale di confine comprende nella fattispecie una zona sita dall’una e dall’altra parte della frontiera italo-svizzera che si estende per circa 10 km dalla frontiera stessa; la procedura per il trasporto dei cadaveri, nell’ambito delle due zone, si applica alle località comprese negli elenchi allegati. 3. I lascia-passare per cadaveri sono rilasciati, da parte svizzera, dalle autorità cantonali e, da parte italiana, dalle autorità provinciali. In Svizzera le autorità competenti sono, per il Cantone dei Grigioni, i com- missariati distrettuali, per il Canton Ticino, le autorità comunali e per il Canton Vallese, la polizia cantonale. In Italia le autorità competenti sono i Prefetti delle Provincie di Como, Va- rese, Sondrio, Novara e il Commissario di Governo della Regione Trentino- Alto Adige2. 4. I lascia-passare per cadaveri non dovranno più essere muniti dell’autentica- zione delle firme.