Research Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MOLLUSCS Species Names – for Consultation 1
MOLLUSCS species names – for consultation English name ‘Standard’ Gaelic name Gen Scientific name Notes Neologisms in italics der MOLLUSC moileasg m MOLLUSCS moileasgan SEASHELL slige mhara f SEASHELLS sligean mara SHELLFISH (singular) maorach m SHELLFISH (plural) maoraich UNIVALVE SHELLFISH aon-mhogalach m (singular) UNIVALVE SHELLFISH aon-mhogalaich (plural) BIVALVE SHELLFISH dà-mhogalach m (singular) BIVALVE SHELLFISH dà-mhogalaich (plural) LIMPET (general) bàirneach f LIMPETS bàirnich common limpet bàirneach chumanta f Patella vulgata ‘common limpet’ slit limpet bàirneach eagach f Emarginula fissura ‘notched limpet’ keyhole limpet bàirneach thollta f Diodora graeca ‘holed limpet’ china limpet bàirneach dhromanach f Patella ulyssiponensis ‘ridged limpet’ blue-rayed limpet copan Moire m Patella pellucida ‘The Virgin Mary’s cup’ tortoiseshell limpet bàirneach riabhach f Testudinalia ‘brindled limpet’ testudinalis white tortoiseshell bàirneach bhàn f Tectura virginea ‘fair limpet’ limpet TOP SHELL brùiteag f TOP SHELLS brùiteagan f painted top brùiteag dhotamain f Calliostoma ‘spinning top shell’ zizyphinum turban top brùiteag thurbain f Gibbula magus ‘turban top shell’ grey top brùiteag liath f Gibbula cineraria ‘grey top shell’ flat top brùiteag thollta f Gibbula umbilicalis ‘holed top shell’ pheasant shell slige easaig f Tricolia pullus ‘pheasant shell’ WINKLE (general) faochag f WINKLES faochagan f banded chink shell faochag chlaiseach bhannach f Lacuna vincta ‘banded grooved winkle’ common winkle faochag chumanta f Littorina littorea ‘common winkle’ rough winkle (group) faochag gharbh f Littorina spp. ‘rough winkle’ small winkle faochag bheag f Melarhaphe neritoides ‘small winkle’ flat winkle (2 species) faochag rèidh f Littorina mariae & L. ‘flat winkle’ 1 MOLLUSCS species names – for consultation littoralis mudsnail (group) seilcheag làthaich f Fam. -

Physiological Responses to Ocean Acidification and Warming
Marine Environmental Research 130 (2017) 38e47 Contents lists available at ScienceDirect Marine Environmental Research journal homepage: www.elsevier.com/locate/marenvrev Physiological responses to ocean acidification and warming synergistically reduce condition of the common cockle Cerastoderma edule * E.Z. Ong a, b, , M. Briffa b, T. Moens a, C. Van Colen a a Ghent University, Biology Department, Marine Biology Research Group, Krijgslaan 281eS8, B 9000 Ghent, Belgium b Marine Biology & Ecology Research Centre, Plymouth University, Plymouth PL4 8AA, UK article info abstract Article history: The combined effect of ocean acidification and warming on the common cockle Cerastoderma edule was Received 4 February 2017 investigated in a fully crossed laboratory experiment. Survival of the examined adult organisms remained Received in revised form high and was not affected by elevated temperature (þ3 C) or lowered pH (À0.3 units). However, the 6 June 2017 morphometric condition index of the cockles incubated under high pCO conditions (i.e. combined Accepted 3 July 2017 2 warming and acidification) was significantly reduced after six weeks of incubation. Respiration rates Available online 5 July 2017 increased significantly under low pH, with highest rates measured under combined warm and low pH conditions. Calcification decreased significantly under low pH while clearance rates increased signifi- Keywords: Future ocean cantly under warm conditions and were generally lower in low pH treatments. The observed physio- fi Ocean acidification logical responses suggest that the reduced food intake under hypercapnia is insuf cient to support the Ocean warming higher energy requirements to compensate for the higher costs for basal maintenance and growth in Cerastoderma edule future high pCO2 waters. -

Mercury Concentration in Bivalve Molluscs
Bull Vet Inst Pulawy 59, 357-360, 2015 DOI:10.1515/bvip-2015-0053 Mercury concentration in bivalve molluscs Józef Szkoda1, Maciej Durkalec1, Agnieszka Nawrocka1, Mirosław Michalski2 1Department of Pharmacology and Toxicology, 2Department of Hygiene of Animal Origin, National Veterinary Research Institute, 24-100 Pulawy, Poland [email protected] Received: April 23, 2015 Accepted: September 4, 2015 Abstract A total of 85 mussel samples of eight species were examined. Analysis of mercury in the freeze-dried samples was carried out by atomic absorption spectrometry method using direct mercury analyser AMA 254. The analytical procedure for determination of mercury was covered by the quality assurance programme of research and participation in national and international proficiency tests. Concentrations of total mercury in all investigated samples were found to be generally low, in the range of 0.033-0.577 mg/kg of dry weight and of 0.003-0.045 mg/kg of wet weight. The results indicate that obtained levels of mercury in bivalve molluscs are not likely to pose a risk to the health of consumers. Keywords: mercury, bivalve molluscs, bioaccumulation, food safety. Introduction which may pose a threat to the health of consumers (7, 13). In Poland, the interest in marine food, or seafood, The rules for the marketing of live bivalve has increased in the recent years. Crustaceans, mostly molluscs for direct consumption and also for shrimps, squids, and lobsters, are frequently chosen processing are strictly defined in EU law regulating by consumers, but less popular oysters, cockles, all aspects of production, including environmental mussels, and clams which belong to bivalve molluscs conditions, farming, cleaning, transport, sale, are also purchased. -
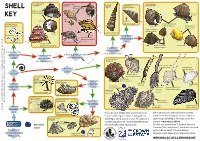
Shell Whelk Dog Whelk Turret It Could Be a Periwinkle Shell (Nucella Lapillus) Shell Spire Shell Thick Top Shell (Osilinus Lineatus) Dark Stripes Key on Body
It could be a type of It could be a type of It could be a It could be a type of topshell whelk Dog whelk turret It could be a periwinkle Shell (Nucella lapillus) shell spire shell Thick top shell (Osilinus lineatus) Dark stripes Key on body Egg Underside capsules Actual size It could be a type of (Hydrobia sp) Common periwinkle spiral worm White ‘Colar’ (Littorina littorea) Flat periwinkle (Littorinasp) Yes Roughly ‘ribbed’ shell. Very high up shore ‘Tooth inside (Turitella communis) opening (Spirorbis sp) Does it have 6 Common whelk No (Buccinum undatum) Yes or more whorls Brown, speckled Netted dog whelk body (twists)? Painted topshell (Nassarius reticulatus) (Calliostoma zizyphinum) No Rough periwinkle Flattened spire Yes Is it long, thin (Littorina saxatilis) Yes Yes and cone shaped Is it permanently No like a unicorn’s horn? attached to Is there a groove or teeth No Is there mother No a surface? in the shell opening? of pearl inside It could be a type of the shell opening? bivalve Yes Yes Common otter-shell (Lutraria lutraria) Bean-like tellin No Is the shell in (Fabulina fabula) Is it 2 parts? spiraled? Common cockle (Cerastoderma edule) It could be a Flat, rounded No sand No Great scallop mason It could be a Is the shell a (Pecten maximus) shell Razor shell worm keel worm Wedge-shaped Is the case dome or (Ensis sp) No Pacific oyster shell made from Yes cone shape? (Crassostrea gigas) Shell can be Peppery furrow shell very large (Scrobicularia plana) sand grains? Elongated and and doesn’t (Lanice conchilega) deep-bodied fully close with large ‘frills’ No (Pomatoceros sp) Yes It could be a type of sea urchin It could be a type of An acorn Native oyster Empty barnacle barnacle Does it have that may be found in estuaries and shores in the UK. -

Impact of Trematode Parasitism on the Fauna of a North Sea Tidal Flat
HELGOI~NDER MEERESUNTERSUCHUNGEN Helgol~nder Meeresunters. 37, 185-199 (1984) Impact of trematode parasitism on the fauna of a North Sea tidal flat G. Lauckner Biologische Anstalt Helgoland (Litoralstation]; D-2282 List/Sylt, Federal Republic of Germany ABSTRACT: The impact of larval trematodes on the fauna of a North Sea tidal flat is considered at the individual and at the population level, depicting the digenean parasites of the common periwinkle, Littorina littorea, and their life cycles, as an example. On the German North Sea coast, L. fittorea is first intermediate host for 6 larval trematodes representing 6 digenean families - Cryptocotyle lingua (Heterophyidae), Himasthla elongata (Echinostomatidae), Renicola roscovita (Renicolidae), Microphallus pygmaeus (Microphallidae), Podocotyle atomon (Opecoelidae} and Cercaria lebouri (Notocotylidae). All except P. atomon utilize shore birds as final hosts; adult P. atomon parasitize in the intestine of teleosts, mainly pleuronectid flatfish. Second intermediate hosts of C. lingua are various species of fish; the cercariae of H. elongata encyst in molluscs and polychaetes, those of R. roscovita in molluscs; Iv[. pygmaeus has an abbreviated life cycle; C. lebouri encysts free on solid surfaces; and the fish trematode P. atomon utilizes benthic crustaceans, mainly amphipods, as second intermediate hosts. On the tidal flats of the K6nigshafen (Sylt), up to 77 % of the periwinkles have been found to be infested by larval trematodes. Maximum infestations in individual samples were 23 % for C. lingua, 47 % for H. etongata and 44 To for R. roscovita. The digeneans cause complete 'parasitic castration' of their carriers and hence exclude a considerable proportion of the snails from the breeding population. -

Solent Bivalve Survey 2019 Report
Solent Bivalve Stock Survey Spring 2019 This report has been produced by the Southern Inshore Fisheries and Conservation Authority. A copy of this report is available on our website at www.southern-ifca.gov.uk or from the Southern IFCA Office at: Unit 3, Holes Bay Park Sterte Road West Poole Dorset BH15 2AA 01202 721373 [email protected] !1 Contents 1. Introduction 3 1.1. The Fishery 3 1.2. The Solent 4 1.3. Current Management 5 2. Methodology 5 2.1. The Survey 5 2.2. Equipment 6 2.3. Data Analysis 6 3. Results 6 3.1. Southampton Water 7 3.2. Portsmouth Harbour 9 3.3. Langstone Harbour 11 4. Discussion 13 4.1. Southampton Water 13 4.2. Portsmouth Harbour 14 4.3. Langstone Harbour 15 4.4. Cockle 16 4.5. American Hard-Shelled clam 17 5. References 17 6. Annex 18 6.1. Annex 1 18 6.2. Annex 2 21 !2 1. Introduction The following report details the bivalve 1 surveys carried out in Southampton Water, Portsmouth Harbour and Langstone Harbour during March - April 2018, October 2018, and March 2019. The report will assess the distribution and abundance of clam and cockle species over time to evaluate the population health and stability of commercially important species for the dredge fishery. In addition, the outcomes from the survey will provide data which can be used as a baseline on which to monitor 2 future trends and potential changes to populations which will feed into the development and monitoring of local management strategies. -

BROODSTOCK CONDITIONING and LARVAL REARING of the GEODUCK CLAM (Panopea Generosa GOULD, 1850)
BROODSTOCK CONDITIONING AND LARVAL REARING OF THE GEODUCK CLAM (Panopea generosa GOULD, 1850) by Robert Marshall B.Sc.(hons), Dalhousie University, 1993 M.Aq., Simon Fraser University, 1997 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in The Faculty of Graduate Studies (Animal Science) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) March 2012 © Robert Marshall, 2012 Abstract The aim of this thesis was to identify conditions that optimize Panopea generosa broodstock conditioning and larval growth and survival in a hatchery setting. A series of experiments subjected broodstock (adults) to various levels of key factors [i.e. temperature (Ch. 2), salinity (Ch. 3) and nutrition [ration (Ch. 4) and feed type (Ch. 5)]. A larval experiment examined the effects of stocking density and feed level combinations on growth and survival (Ch. 6). Broodstock responses were quantified using gravimetric (condition and gonadosomatic indices) and histological techniques (development classification, volume fractions and oocyte diameter). Survival and spawning rates were also examined. Of the temperatures tested (7, 11, 15 and 19˚C) 11˚C had the highest spawning rates (% individuals) and more oocytes follicle-1, than 15 and 19˚C. At 7˚C gonadosomatic indices were highest but this temperature did not produce spawning clams. Gonads degenerated at 19˚C. Among salinities of 17, 20, 24, and 29 gonad sheath thickness and area occupied by gametes increased at 29 but not at 24. Salinities of 17 and 20 were associated with fungal infection and had high mortality rates after 26 d exposure. With higher ration treatments (up to 7.2 × 109 cells clam-1 d-1 [Isochrysis sp. -

Biogeographic Patterns of the Marine Bivalve Cerastoderma Edule Along European Atlantic Coasts
Biogeographic patterns of the marine bivalve Cerastoderma edule along European Atlantic coasts DISSERTATION Zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Christian-Albrecht-Universität zu Kiel vorgelegt von Manuela Krakau Kiel 2008 Angefertigt an der Wattenmeerstation Sylt Stiftung Alfred-Wegener-Institut für Polar- und Meeresforschung Referent: Prof. Dr. Karsten Reise Koreferent: Prof. Dr. Reinhold Hanel Tag der mündlichen Prüfung: 10. Juli 2008 Zum Druck genehmigt: 10. Juli 2008 CONTENTS SUMMARY ...…...………………………………….………………………… I ZUSAMMENFASSUNG .……………………………………………………... III GENERAL INTRODUCTION .………………………………………………….. 1 CHAPTER 1: Shell forms of the intertidal bivalve Cerastoderma edule L. from Africa to the Arctic .………………………………………… 9 CHAPTER 2: Cockle parasites across biogeographic provinces ..……......… 36 CHAPTER 3: Genetic diversity in high latitudes – an intertidal bivalve contradicts a common pattern ..…………………………… 59 GENERAL DISCUSSION .…………………...……………………………….. 89 REFERENCES …..…………………………………………………………... 95 APPENDIX ………………………………………………………………… X1 ACKNOWLEDGEMENTS /D ANKSAGUNG Biogeographic patterns of the marine bivalve C. edule SUMMARY SUMMARY The cockle Cerastoderma edule is a common bivalve that inhabits the marine soft-bottom intertidal along European shores. This invertebrate plays a key role in coastal food webs of the Northeast Atlantic coasts due of its high abundances. I studied cockles from 19 sites along the distribution range with the aim to describe the variation of geographic population -

Alaska Final Report
Final Report Chugach Regional Resources Commission Bivalve Enhancement Program Bivalve inventories and native littleneck clam (Protothaca staminea) culture studies Exxon Valdez Oil Spill Trustee Council Project Number 95131 Produced by: Dr. Kenneth M. Brooks Aquatic Environmental Sciences 644 Old Eaglemount Road Port Townsend, Washington 98368 February 2, 2001 Chugach Regional Resources Commission Bivalve Enhancement Program – Bivalve Inventories and native littleneck clam (Protothaca staminea) culture studies Table of contents Page Introduction 1 1.0. Background information 2 1.1. Littleneck clam life history 2 1.1.1. Reproduction 3 1.1.2. Distribution as a function of tidal elevation. 3 1.1.3. Substrate preferences 3 1.1.4. Habitat Suitability Index (HIS) for native littleneck clams. 3 1.2. Marking clams and other bivalves 5 1.3. Aging of bivalves 6 1.4. Length at age for native littleneck clams in Alaska 8 1.5. Bivalve predators 8 1.6. Bivalve culture 9 1.7. Clam culture techniques 10 1.7.1. Predator control 11 1.7.2. Supplemental seeding 11 1.7.3. Substrate modification. 11 1.7.4. Plastic netting 11 1.7.5. Plastic clam bags 12 1.8. Commercial clam harvest management in Alaska 12 1.9. Environmental effects associated with bivalve culture 13 1.10. Background summary 15 1.11. Purpose of this study 15 2.0. Materials, methods and results for the bivalve inventories conducted in 1995 and 1996 at Port Graham, Nanwalek, Tatitlek, Chenega and Ouzinke. 17 2.1. 1995-96 bivalve inventory sampling design. 17 2.2. Clam sample processing. 18 2.3. -

Inshore Fisheries: Bivalve Molluscs
Inshore Fisheries: Bivalve Molluscs Scallops Due to a rapid growth rate and high market value, the scallop fishery is of high economic importance. Fishing for scallops has been established in Northern Ireland since 1935 with Queen scallop fishing not becoming commercial until the 1970’s. Due to the different behaviour of King and Queen scallops, with King scallops burying into the sediment whilst Queen scallops are active swimmers, the fishing technique changes to encompass this. King scallops are fished using dredges with 8 or 9 metal teeth set vertically along the front edge of the dredge. The teeth rake up the scallops which are caught by the mesh bag positioned behind the tooth bar. Groups of dredges are hung from a tow bar which has wheels on either end so it can move over the seabed. As Queen scallops tend to swim when disturbed, fishermen can make use of this by using skid dredges or otter trawls. Unlike scallop dredges, skid dredges have no teeth, but instead have a tickler chain which disturbs the Queen scallops causing them to swim into the water column where they are caught by the net attached behind the tickler chain. Management All vessels fishing scallops have to submit shellfish landings to the Department of Agriculture and Rural Development. Information submitted includes port of landing, length of vessel, live weight and value. In addition, all vessels over 10m which harvest scallops have to have a vessel monitoring system on board. In 2008 new regulations came out under the Conservation of Scallop Regulations (Northern Ireland) 2008 which replace the Conservation of Scallops Regulations (Northern Ireland) 1997 and The Inshore Fishing (Daily Closed Time for Scallops) Regulations (Northern Ireland) 2000. -

CRRC Bivalve and Littleneck Clam Culture Studies
5.0. Development of hatchery, nursery and growout methods for Nuttall’s cockle (Clinocardium nuttallii). During the 1995 shellfish surveys at the Alaskan Native villages of Tatitlek, Port Graham and Nanwalek, villagers repeatedly expressed a preference for cockles (Clinocardium nuttallii). Residents of Port Graham reported that cockles were common in the 1970’s and early 1980’s, but virtually disappeared several years before the Exxon Valdez oil spill. Very few cockles were observed in any of the quantitative or qualitative surveys conducted at Port Graham, Tatitlek, or Nanwalek. Excellent cockle habitat was observed in qualitative shellfish surveys at Port Graham and Tatitlek. The common cockle from the Eastern Atlantic (Cerastoderma edule) is prized in some areas of Europe and blood cockles of the genus Anadara are grown and marketed in Asia. However, Nuttall’s cockle, common in sandy intertidal areas of the eastern Pacific, is not cultivated and is not commonly harvested commercially. In part, that is because this bivalve does not keep well under refrigeration (author’s personal experience) and therefore has a limited commercial shelf-life. The result is that little work has been accomplished with respect to developing hatchery techniques for propagating this animal. A search of the ASFA and BIOSYS bibliographic databases revealed few citations dealing with the genus Clinocardium. All of those identified in the search were obtained from the University of Washington library system together with many of the references pertaining to other cockle species. 5.1. Background. In addition to being a favored food of Alaskan Natives, cockles appear to grow rapidly in Washington State. -

104 Th Annual Meeting, Seattle, Washington, March 2012
Journal of Shellfish Research, Vol. 31, No. 1, 231, 2012. ABSTRACTS OF TECHNICAL PAPERS Presented at the 104th Annual Meeting NATIONAL SHELLFISHERIES ASSOCIATION Seattle, Washington March 24–29, 2012 231 National Shellfisheries Association, Seattle, Washington Abstracts 104th Annual Meeting, March 24–29, 2012 233 CONTENTS Alisha Aagesen, Chris Langdon, Claudia Hase AN ANALYSIS OF TYPE IV PILI IN VIBRIO PARAHAEMOLYTICUS AND THEIR INVOLVEMENT IN PACIFICOYSTERCOLONIZATION........................................................... 257 Cathryn L. Abbott, Nicolas Corradi, Gary Meyer, Fabien Burki, Stewart C. Johnson, Patrick Keeling MULTIPLE GENE SEGMENTS ISOLATED BY NEXT-GENERATION SEQUENCING INDICATE EXTREME DIVERGENCE OF MIKROCYTOS MACKINI. ......................................... 257 Cathryn L. Abbott, Scott R. Gilmore, Geoff Lowe, Gary Meyer, Susan Bower A JOURNEY TOWARDS MOLECULAR DIAGNOSTICS FOR AN UNCLASSIFIABLE INTRACELLULAR MICROCELL PARASITE OF PACIFIC OYSTERS (CRASSOSTREA GIGAS), MIKROCYTOS MACKINI. .................................................................... 257 Robert R. Abbott, Rena Obernolte ACOUSTICALLY TAGGED FISH UTILIZATION OF AN ARTIFICIAL REEF CONSTRUCTED FOR NATIVEOLYMPIAOYSTERRESTORATION................................................... 257 Kumud Acharya, Lynn Schwaebe, Michael Nicholl WHAT INDUCES DREISSENA BUGENSIS TO SPAWN UNDER LABORATORY CONDITIONS? . 258 Chuck Adams, Leslie Sturmer AN ECONOMIC DESCRIPTION OF THE FLORIDA SHELLFISH CULTURE INDUSTRY. 258 Justin Ainsworth, Mitch Vance ESTIMATING DISCARD RATES