CRRC Bivalve and Littleneck Clam Culture Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
UNITED NATIONS ENVIRONMENT PROGRAM MEDITERRANEAN ACTION PLAN REGIONAL ACTIVITY CENTRE FOR SPECIALLY PROTECTED AREAS National monitoring program for biodiversity and non-indigenous species in Egypt PROF. MOUSTAFA M. FOUDA April 2017 1 Study required and financed by: Regional Activity Centre for Specially Protected Areas Boulevard du Leader Yasser Arafat BP 337 1080 Tunis Cedex – Tunisie Responsible of the study: Mehdi Aissi, EcApMEDII Programme officer In charge of the study: Prof. Moustafa M. Fouda Mr. Mohamed Said Abdelwarith Mr. Mahmoud Fawzy Kamel Ministry of Environment, Egyptian Environmental Affairs Agency (EEAA) With the participation of: Name, qualification and original institution of all the participants in the study (field mission or participation of national institutions) 2 TABLE OF CONTENTS page Acknowledgements 4 Preamble 5 Chapter 1: Introduction 9 Chapter 2: Institutional and regulatory aspects 40 Chapter 3: Scientific Aspects 49 Chapter 4: Development of monitoring program 59 Chapter 5: Existing Monitoring Program in Egypt 91 1. Monitoring program for habitat mapping 103 2. Marine MAMMALS monitoring program 109 3. Marine Turtles Monitoring Program 115 4. Monitoring Program for Seabirds 118 5. Non-Indigenous Species Monitoring Program 123 Chapter 6: Implementation / Operational Plan 131 Selected References 133 Annexes 143 3 AKNOWLEGEMENTS We would like to thank RAC/ SPA and EU for providing financial and technical assistances to prepare this monitoring programme. The preparation of this programme was the result of several contacts and interviews with many stakeholders from Government, research institutions, NGOs and fishermen. The author would like to express thanks to all for their support. In addition; we would like to acknowledge all participants who attended the workshop and represented the following institutions: 1. -

WHELKS Scientific Names: Busycon Canaliculatum Busycon Carica
Colloquial Nicknames: Channeled Whelk Knobbed Whelk WHELKS Scientific names: Busycon canaliculatum Busycon carica Field Markings: The shell of open with their strong muscular foot. As both species is yellow-red or soon as the valves open, even the tiniest orange inside and pale gray amount, the whelk wedges in the sharp edge outside. of its shell, inserts the proboscis and Size: Channeled whelk grows up devours the soft body of the clam. to 8 inches long; knobbed whelk Mating occurs by way of internal grows up to 9 inches long and 4.5 inches wide fertilization; sexes are separate. The egg casing of the whelk is a Habitat: Sandy or muddy bottoms long strand of yellowish, parchment-like disks, resembling a Seasonal Appearance: Year-round necklace - its unique shape is sculpted by the whelk’s foot. Egg cases can be two to three feet long and have 70 to 100 capsules, DISTINGUISHING FEATURES AND each of which can hold 20 to 100 eggs. Newly hatched channeled BEHAVIORS whelks escape from small holes at the top of each egg case with Whelks are large snails with massive shells. The two most their shells already on. Egg cases are sometimes found along common species in Narragansett Bay are the knobbed whelk the Bay shoreline, washed up with the high tide debris. and the channeled whelk. The knobbed whelk is the largest marine snail in the Bay. It Relationship to People is pear-shaped with a flared outer lip and knobs on the shoulder Both channeled and knobbed whelks scavenge and hunt for of its shell. -

Age Structure, Growth and Shell Form of Cerastoderma Glaucum (Bivalvia
Age structure, growth and shell form of Cerastoderma glaucum (Bivalvia: Cardiidae) from El Mellah lagoon, Algeria Lamia Bensaâd-Bendjedid, Saber Belhaoues, Asma Kerdoussi, Naouel Djebbari, Mardja Tahri, Mourad Bensouilah Laboratory of Ecobiology for Marine Environment and Coastlines, Faculty of Science, Badji Mokhtar University, Annaba 23000, Algeria. Corresponding author: L. Bensaâd-Benjedid, [email protected] Abstract. Cerastoderma glaucum (Poiret, 1789) the lagoon specialist cockle, represents one of the most abundant molluscs in El Mellah coastal lagoon (northeastern Algeria); several morphological and biological characteristics of this species were investigated over a period of 18 months (January 2014 to June 2015). Analysis of allometry and different morphometric indices indicate that this population tends to assume a globular shell shape which becomes elongated as the cockle grows. From length-frequency data examination using software FiSAT II and VONBIT for Excel, age was ranged from the first to the third year old, asymptotic length (L∞) was estimated to 48.061 mm and growth coefficient (K) was 0.71 -1 yr . The growth performance index (Φ′) and theoretical life span (tmax) were 3.21 and 4.23 yr respectively, total mortality rate (Z) obtained was 1.94 yr-1. Monthly variations in condition index were noticed, two sharp falls due spawning events were observed, the first spawning occurred between March and June and, the second one took place from August to October. The findings of the current study can be utilized to guide future research in various fields as pathology, biomonitoring and environmental management of this species. Key Words: Cerastoderma glaucum, shell shape, growth, Condition index, Algeria. -

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -

Weekend Seafood Feast LINK All Day Dining
Weekend Seafood Feast LINK all day dining Menu 1 Marinated Salad Cucumber mint leave ,cold potato soup,crab meat and coleslaw,sea food calipso , Tuna & potato salad, grilled vegetables and tomato comfit balsamic reduction, smoked salmon and cream cheese, red capsicum and garlic flake salad,tuna and arugula roll,Beetroot and seafood Dressings & Vinaigrettes GMB Caesar dressing, 1000 island, balsamique dressing ,lemon garlic vinaigrette,orange oil, mustard rosemary vinaigrette, cucumber dressing, curry vinaigrette, pesto vinaigrette, Cold Cut Smoked salmon, smoked mackerel,Smoked tenggiri Japanese Sections Chef Choice of sushi and sashimi Salmon, Salmon maki, Tempura maki Spicy crab roll,Octopus gunkan,Salmon sushi Condiment LINK Wasabi, soya sauce, ginger pickles Cold Seafood on ice (imported Seafood/ Subject to change) LINK Green mussel, yabbies’ ’Alaskan king crab leg, Poached tiger prawn, slipper lobster, clam, 1/2 shell scallop, Fresh oyster, Blue crab on ice, cockle Condiment Cocktail sauce, shallot vinegar, lemon Cheese platter 3 type of hard and soft cheese Cheese Condiment Cream Cracker, Apricot, Exotic dried Mix, Cashew Almond,Muesli Red / White Chef Live Action Noodle Section Mee rebus Slice beef,Bean sprout,Sawi,Boiled Egg Fried bean curd,Slice Green Chilli Slice Red Chilli,Fried Shallot Spring onion,Coriander Lime Clear seafood Soup Bean sprout,Sawi,Tiger prawn,clam Inoki mushroom,Spring onion Fried shallot Condiments Chili kicap, green chili pickle Gohan Assorted Tempura Shrimp, oyster mushroom and assorted vegetable Action -

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Lagoon cockle (Cerastoderma glaucum) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Nicola White 2002-07-15 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1315]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: White, N. 2002. Cerastoderma glaucum Lagoon cockle. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1315.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2002-07-15 Lagoon cockle (Cerastoderma glaucum) - Marine Life Information Network See online review for distribution map Three Cerastoderma glaucum with siphons extended. Distribution data supplied by the Ocean Photographer: Dennis R. -
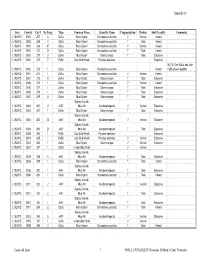
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

ED360998.Pdf
DOCUMENT RESUME ED 360 998 IR 054 690 AUTHOR Byrne, Sherry TITLE Collection Maintenance and Improvement. INSTITUTION Association of Research Libraries, Washington, D.C. SPONS AGENCY National Ene)wment for the Humanities (NFAH), Washington, D.C. REPORT NO ISBN-0-918006-62-7 PUB DATE Jan 93 NOTE 205p.; "One of seven in a series of Preservation Planning Program (PPP) resource guides" (Jutta Reed-Scott, Series Editor), see IR 054 689-695. AVAILABLE FROMAssociation of Research Libraries, 21 Dupont Circle, N.W., Suite 800, Washington, DC 20036. PUB TYPE Collected Works General !020) Guides Non-Classroom Use (055) Reports Evaluative/Feasibility (142) EDRS PRICE MF01/PC09 Plus Postage. DESCRIPTORS Academic Libraries; *Archives; Audiotape Recordings; Books; Evaluation Methods; Higher Education; Librarians; Library Collection Development; *Library Collections; *Library Materials; Library Technicians; *Maintenance; Microforms; Photographs; *Preservation; Program Development; Records Management; Research Libraries; Resou..ce Materials IDENTIFIERS Book Restoration; *Library Materials Conservation ABSTRACT This resource guide provides information about the range of activities that can be implemented to maintain and improve the condition of research collections to ensure that they remain usable as long as possible. After an introduction that describes the major activities and a review of an investigation process that gives an overview of good practice, the following articles are presented: (1) "Handling Books in General Collections" (Library of Congress); -

Data From: Microplastic Concentrations in Two Oregon Bivalve Species: Spatial, Temporal, and Species Variability
Portland State University PDXScholar Environmental Science and Management Datasets Environmental Science and Management 7-2019 Data From: Microplastic Concentrations in Two Oregon Bivalve Species: Spatial, Temporal, and Species Variability Britta Baechler Portland State University, [email protected] Elise F. Granek Portland State University, [email protected] Matthew V. Hunter Oregon Department of Fish and Wildlife Kathleen E. Conn United States Geological Survey Follow this and additional works at: https://pdxscholar.library.pdx.edu/esm_data Part of the Environmental Health and Protection Commons, Environmental Indicators and Impact Assessment Commons, and the Environmental Monitoring Commons Let us know how access to this document benefits ou.y Recommended Citation Baechler, Britta; Granek, Elise F.; Hunter, Matthew V.; and Conn, Kathleen E., "Microplastic Concentrations in Two Oregon Bivalve Species: Spatial, Temporal, and Species Variability" (2019). [Dataset]. https://doi.org/10.15760/esm-data.1 This Dataset is brought to you for free and open access. It has been accepted for inclusion in Environmental Science and Management Datasets by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. Metadata template1 for datasets of L&O-Letters articles Table 1. Description of the fields needed to describe the creation of your dataset. Title of dataset Microplastic Concentrations in Two Oregon Bivalve Species: Spatial, Temporal, and Species Variability URL of dataset Data is available in the Portland State University PDXScholar data repository at: https://doi.org/10.15760/esm-data.1 Abstract Microplastics are an ecological stressor with implications for ecosystem and human health when present in seafood. -

THE NAUTILUS [Vol
2 2 THE NAUTILUS [Vol. 69 (1) separated from each other by five centimeters. The snail was expanded with its head oriented away from the clam. When placed on the sand, the clam showed no activity during the next 30 minutes after which observations were discontinued. All but the lower (anterior) end of the body of the clam was covered by an envelope of slime secreted by the foot of the snail. It seems clear that P. duplicatus capturesEnsis directus by approaching it below the surface of the substratum and by ir ritating the lower portion so that it retreats upward. The snail then coats the razor clam with an envelope of slime which ap pears to have anesthetic properties. Successful capture proba bly depends on the ability of the snail to maintain contact with its prey until anesthesia takes place. ON THE OCCURRENCE OF THE NUDIBRANCH ALDERIA MODESTA (LOVÉN, 1844) ON THE CENTRAL CALIFORNIAN COAST By CADET HAND and JOAN STEINBERG Department of Zoology, University of California, Berkeley Alderia modesta (Loven, 1844) has long been known from the coasts of northern Europe. It has been recorded from as far north as the Trondheim Fjord in Norway (Norman, 1893), south to Skibbereen in Ireland (Allman, 1845) and on the French coast (Gollien, 1929). Therefore, it has been of con siderable interest to us to find well-established populations of an Alderia in two localities on the central Californian coast. Through the kindness of Monsieur G. Van Put of the Royal Institute of Natural Sciences of Belgium in Brussels and Dr. -

Shellfish Hatchery
EAST HAMPTON TOWN SHELLFISH HATCHERY The 2015 Crew, left to right: Kate, Pete, Carissa, Shelby, and Barley 2015 ANNUAL REPORT AND 2016 OPERATING PLAN Prepared by Kate Rossi-Snook Edited by Barley Dunne East Hampton Town Shellfish Hatchery The skiff loaded for seeding in Lake Montauk Annual Report of Operations Mission Statement With a hatchery on Fort Pond Bay, a nursery on Three Mile Harbor, and a floating raft field growout system in Napeague Harbor, the East Hampton Town Shellfish Hatchery produces large quantities of oyster (Crassostrea virginica), clam (Mercenaria mercenaria), and bay scallop (Argopecten irradians) seed to enhance valuable shellfish stocks in local waterways. Shellfish are available for harvest by all permitted town residents. Cooperative research and experimentation concerning shellfish culture, the subsequent success of seed in the wild, and the status of the resource is undertaken and reported upon regularly, often funded and validated by scientific research grants. Educational opportunities afforded by the work include school group and open house tours and educational displays at community functions. Annual reporting includes production statistics and values, seed dissemination information, results of research initiatives, a summary of outreach efforts, the status of current and developing infrastructure, and a plan for the following year’s operations. 2015 Full-time Staff Part-time and Contractual Volunteers John “Barley” Dunne – Director Carissa Maurin – Environmental Aide Romy Macari Kate Rossi-Snook – Hatchery Manager Shelby Joyce – Environmental Aide (summer) Christopher Fox-Strauss Pete Topping – Algae Culturist Adam Younes – Environmental Aide (fall) Jeremy Gould – Maintenance Mechanic Carissa and Pete unloading OysterGros Special Thanks to: Barnaby Friedman for producing our annual seeding maps. -

Shellfish Regulations
Town of Nantucket Shellfishing Policy and Regulations As Adopted on March 4, 2015 by Nantucket Board of Selectmen Amended March 23, 2016; Amended April 20, 2016 Under Authority of Massachusetts General Law, Chapter 130 Under Authority of Chapter 122 of the Code of the Town of Nantucket TABLE OF CONTENTS Section 1 – Shellfishing Policy for the Town of Nantucket/Purpose of Regulations Section 2 – General Regulations (Applying to Recreational, Commercial and Aquaculture Licenses) 2.1 - License or Permit Required 2.2 - Areas Where Recreational or Commercial Shellfishing May Occur 2.3 - Daily Limit 2.4 - Landing Shellfish 2.5 - Daily Time Limit 2.6 - Closures and Red Flag 2.7 - Temperature Restrictions 2.8 - Habitat Sensitive Areas 2.9 - Bay Scallop Strandings 2.10 - Poaching 2.11 - Disturbance of Licensed or Closed Areas 2.12 - Inspection on Demand 2.13 - Possession of Seed 2.14 - Methods of Taking 2.15 – SCUBA Diving and Snorkeling 2.16 - Transplanting, Shipping, and Storing of Live Shellfish 2.16a - Transplanting Shellfish Outside Town Waters 2.16b - Shipping of Live Shellfish for Broodstock Purposes 2.16c - Transplanting Shellfish into Town Waters 2.16d - Harvesting Seed from the Wild Not Allowed 2.16e - Wet Storage of Recreational Shellfish Prohibited. 2.17 - By-Catch 2.18 - Catch Reports Provided to the Town 2.18a - Commercial Catch Reports 2.18b - Recreational Catch Reports Section 3 – Recreational (Non-commercial) Shellfishing 3.1 - Permits 3.1a - No Transfers or Refunds 3.1b - Recreational License Fees 3.2 - Cannot Harvest for Commerce