82 Undersized Clams Planted In1997
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -
Copy of Appendix 5 Catalogue
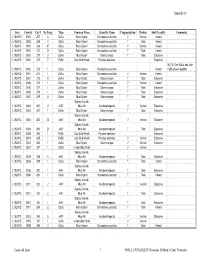
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2013): 4.438 New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India Souji.S1, Tresa Radhakrishnan2 Department of Aquatic Biology and Fisheries, University of Kerala, Thiruvananthapuram-695 581, Kariyavattom, Kerala, India Abstract: Arcacea family is economically important group of animals. Most of the species in this family are misplaced into invalid subgenera and Indian arcids are wanted a revision in systematic positon. In the case of Arcidae family; all of the species are treated under Anadara as main genera, however, some authors considered that the Tegillarca genus is only a sub genus of Arcidae family. Anadara is the commercially important genus of bivalves of Arcidae family. These two genera are confused by many taxonomists and some considered that the morphometric changes of Tegillarca are only the habitual adaptation. But the collected samples from the same habitat from the southern part of India is clearly demarked the distinction between Anadara species and Tegillarca species. In this paper the differences between these two genera are illustrated with the help of specimens from the same habitat and with the help of taxonomic literature of these genera. Species level classification was done based on the morphometric characters like peculiarities of (i) periostracum, (ii) cardinal area, (iii) umbo, (iv) adductor muscle scar and (v) pallial line. The specimens were collected from Neendakara, Vizhinjam and Kovalam along with the south west coast and Thiruchendur in Tamil Nadu, south east coast of India. -

MOLLUSCS Species Names – for Consultation 1
MOLLUSCS species names – for consultation English name ‘Standard’ Gaelic name Gen Scientific name Notes Neologisms in italics der MOLLUSC moileasg m MOLLUSCS moileasgan SEASHELL slige mhara f SEASHELLS sligean mara SHELLFISH (singular) maorach m SHELLFISH (plural) maoraich UNIVALVE SHELLFISH aon-mhogalach m (singular) UNIVALVE SHELLFISH aon-mhogalaich (plural) BIVALVE SHELLFISH dà-mhogalach m (singular) BIVALVE SHELLFISH dà-mhogalaich (plural) LIMPET (general) bàirneach f LIMPETS bàirnich common limpet bàirneach chumanta f Patella vulgata ‘common limpet’ slit limpet bàirneach eagach f Emarginula fissura ‘notched limpet’ keyhole limpet bàirneach thollta f Diodora graeca ‘holed limpet’ china limpet bàirneach dhromanach f Patella ulyssiponensis ‘ridged limpet’ blue-rayed limpet copan Moire m Patella pellucida ‘The Virgin Mary’s cup’ tortoiseshell limpet bàirneach riabhach f Testudinalia ‘brindled limpet’ testudinalis white tortoiseshell bàirneach bhàn f Tectura virginea ‘fair limpet’ limpet TOP SHELL brùiteag f TOP SHELLS brùiteagan f painted top brùiteag dhotamain f Calliostoma ‘spinning top shell’ zizyphinum turban top brùiteag thurbain f Gibbula magus ‘turban top shell’ grey top brùiteag liath f Gibbula cineraria ‘grey top shell’ flat top brùiteag thollta f Gibbula umbilicalis ‘holed top shell’ pheasant shell slige easaig f Tricolia pullus ‘pheasant shell’ WINKLE (general) faochag f WINKLES faochagan f banded chink shell faochag chlaiseach bhannach f Lacuna vincta ‘banded grooved winkle’ common winkle faochag chumanta f Littorina littorea ‘common winkle’ rough winkle (group) faochag gharbh f Littorina spp. ‘rough winkle’ small winkle faochag bheag f Melarhaphe neritoides ‘small winkle’ flat winkle (2 species) faochag rèidh f Littorina mariae & L. ‘flat winkle’ 1 MOLLUSCS species names – for consultation littoralis mudsnail (group) seilcheag làthaich f Fam. -

Physiological Responses to Ocean Acidification and Warming
Marine Environmental Research 130 (2017) 38e47 Contents lists available at ScienceDirect Marine Environmental Research journal homepage: www.elsevier.com/locate/marenvrev Physiological responses to ocean acidification and warming synergistically reduce condition of the common cockle Cerastoderma edule * E.Z. Ong a, b, , M. Briffa b, T. Moens a, C. Van Colen a a Ghent University, Biology Department, Marine Biology Research Group, Krijgslaan 281eS8, B 9000 Ghent, Belgium b Marine Biology & Ecology Research Centre, Plymouth University, Plymouth PL4 8AA, UK article info abstract Article history: The combined effect of ocean acidification and warming on the common cockle Cerastoderma edule was Received 4 February 2017 investigated in a fully crossed laboratory experiment. Survival of the examined adult organisms remained Received in revised form high and was not affected by elevated temperature (þ3 C) or lowered pH (À0.3 units). However, the 6 June 2017 morphometric condition index of the cockles incubated under high pCO conditions (i.e. combined Accepted 3 July 2017 2 warming and acidification) was significantly reduced after six weeks of incubation. Respiration rates Available online 5 July 2017 increased significantly under low pH, with highest rates measured under combined warm and low pH conditions. Calcification decreased significantly under low pH while clearance rates increased signifi- Keywords: Future ocean cantly under warm conditions and were generally lower in low pH treatments. The observed physio- fi Ocean acidification logical responses suggest that the reduced food intake under hypercapnia is insuf cient to support the Ocean warming higher energy requirements to compensate for the higher costs for basal maintenance and growth in Cerastoderma edule future high pCO2 waters. -

Mercury Concentration in Bivalve Molluscs
Bull Vet Inst Pulawy 59, 357-360, 2015 DOI:10.1515/bvip-2015-0053 Mercury concentration in bivalve molluscs Józef Szkoda1, Maciej Durkalec1, Agnieszka Nawrocka1, Mirosław Michalski2 1Department of Pharmacology and Toxicology, 2Department of Hygiene of Animal Origin, National Veterinary Research Institute, 24-100 Pulawy, Poland [email protected] Received: April 23, 2015 Accepted: September 4, 2015 Abstract A total of 85 mussel samples of eight species were examined. Analysis of mercury in the freeze-dried samples was carried out by atomic absorption spectrometry method using direct mercury analyser AMA 254. The analytical procedure for determination of mercury was covered by the quality assurance programme of research and participation in national and international proficiency tests. Concentrations of total mercury in all investigated samples were found to be generally low, in the range of 0.033-0.577 mg/kg of dry weight and of 0.003-0.045 mg/kg of wet weight. The results indicate that obtained levels of mercury in bivalve molluscs are not likely to pose a risk to the health of consumers. Keywords: mercury, bivalve molluscs, bioaccumulation, food safety. Introduction which may pose a threat to the health of consumers (7, 13). In Poland, the interest in marine food, or seafood, The rules for the marketing of live bivalve has increased in the recent years. Crustaceans, mostly molluscs for direct consumption and also for shrimps, squids, and lobsters, are frequently chosen processing are strictly defined in EU law regulating by consumers, but less popular oysters, cockles, all aspects of production, including environmental mussels, and clams which belong to bivalve molluscs conditions, farming, cleaning, transport, sale, are also purchased. -
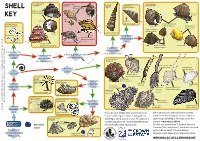
Shell Whelk Dog Whelk Turret It Could Be a Periwinkle Shell (Nucella Lapillus) Shell Spire Shell Thick Top Shell (Osilinus Lineatus) Dark Stripes Key on Body
It could be a type of It could be a type of It could be a It could be a type of topshell whelk Dog whelk turret It could be a periwinkle Shell (Nucella lapillus) shell spire shell Thick top shell (Osilinus lineatus) Dark stripes Key on body Egg Underside capsules Actual size It could be a type of (Hydrobia sp) Common periwinkle spiral worm White ‘Colar’ (Littorina littorea) Flat periwinkle (Littorinasp) Yes Roughly ‘ribbed’ shell. Very high up shore ‘Tooth inside (Turitella communis) opening (Spirorbis sp) Does it have 6 Common whelk No (Buccinum undatum) Yes or more whorls Brown, speckled Netted dog whelk body (twists)? Painted topshell (Nassarius reticulatus) (Calliostoma zizyphinum) No Rough periwinkle Flattened spire Yes Is it long, thin (Littorina saxatilis) Yes Yes and cone shaped Is it permanently No like a unicorn’s horn? attached to Is there a groove or teeth No Is there mother No a surface? in the shell opening? of pearl inside It could be a type of the shell opening? bivalve Yes Yes Common otter-shell (Lutraria lutraria) Bean-like tellin No Is the shell in (Fabulina fabula) Is it 2 parts? spiraled? Common cockle (Cerastoderma edule) It could be a Flat, rounded No sand No Great scallop mason It could be a Is the shell a (Pecten maximus) shell Razor shell worm keel worm Wedge-shaped Is the case dome or (Ensis sp) No Pacific oyster shell made from Yes cone shape? (Crassostrea gigas) Shell can be Peppery furrow shell very large (Scrobicularia plana) sand grains? Elongated and and doesn’t (Lanice conchilega) deep-bodied fully close with large ‘frills’ No (Pomatoceros sp) Yes It could be a type of sea urchin It could be a type of An acorn Native oyster Empty barnacle barnacle Does it have that may be found in estuaries and shores in the UK. -

Impact of Trematode Parasitism on the Fauna of a North Sea Tidal Flat
HELGOI~NDER MEERESUNTERSUCHUNGEN Helgol~nder Meeresunters. 37, 185-199 (1984) Impact of trematode parasitism on the fauna of a North Sea tidal flat G. Lauckner Biologische Anstalt Helgoland (Litoralstation]; D-2282 List/Sylt, Federal Republic of Germany ABSTRACT: The impact of larval trematodes on the fauna of a North Sea tidal flat is considered at the individual and at the population level, depicting the digenean parasites of the common periwinkle, Littorina littorea, and their life cycles, as an example. On the German North Sea coast, L. fittorea is first intermediate host for 6 larval trematodes representing 6 digenean families - Cryptocotyle lingua (Heterophyidae), Himasthla elongata (Echinostomatidae), Renicola roscovita (Renicolidae), Microphallus pygmaeus (Microphallidae), Podocotyle atomon (Opecoelidae} and Cercaria lebouri (Notocotylidae). All except P. atomon utilize shore birds as final hosts; adult P. atomon parasitize in the intestine of teleosts, mainly pleuronectid flatfish. Second intermediate hosts of C. lingua are various species of fish; the cercariae of H. elongata encyst in molluscs and polychaetes, those of R. roscovita in molluscs; Iv[. pygmaeus has an abbreviated life cycle; C. lebouri encysts free on solid surfaces; and the fish trematode P. atomon utilizes benthic crustaceans, mainly amphipods, as second intermediate hosts. On the tidal flats of the K6nigshafen (Sylt), up to 77 % of the periwinkles have been found to be infested by larval trematodes. Maximum infestations in individual samples were 23 % for C. lingua, 47 % for H. etongata and 44 To for R. roscovita. The digeneans cause complete 'parasitic castration' of their carriers and hence exclude a considerable proportion of the snails from the breeding population. -

Solent Bivalve Survey 2019 Report
Solent Bivalve Stock Survey Spring 2019 This report has been produced by the Southern Inshore Fisheries and Conservation Authority. A copy of this report is available on our website at www.southern-ifca.gov.uk or from the Southern IFCA Office at: Unit 3, Holes Bay Park Sterte Road West Poole Dorset BH15 2AA 01202 721373 [email protected] !1 Contents 1. Introduction 3 1.1. The Fishery 3 1.2. The Solent 4 1.3. Current Management 5 2. Methodology 5 2.1. The Survey 5 2.2. Equipment 6 2.3. Data Analysis 6 3. Results 6 3.1. Southampton Water 7 3.2. Portsmouth Harbour 9 3.3. Langstone Harbour 11 4. Discussion 13 4.1. Southampton Water 13 4.2. Portsmouth Harbour 14 4.3. Langstone Harbour 15 4.4. Cockle 16 4.5. American Hard-Shelled clam 17 5. References 17 6. Annex 18 6.1. Annex 1 18 6.2. Annex 2 21 !2 1. Introduction The following report details the bivalve 1 surveys carried out in Southampton Water, Portsmouth Harbour and Langstone Harbour during March - April 2018, October 2018, and March 2019. The report will assess the distribution and abundance of clam and cockle species over time to evaluate the population health and stability of commercially important species for the dredge fishery. In addition, the outcomes from the survey will provide data which can be used as a baseline on which to monitor 2 future trends and potential changes to populations which will feed into the development and monitoring of local management strategies. -

BROODSTOCK CONDITIONING and LARVAL REARING of the GEODUCK CLAM (Panopea Generosa GOULD, 1850)
BROODSTOCK CONDITIONING AND LARVAL REARING OF THE GEODUCK CLAM (Panopea generosa GOULD, 1850) by Robert Marshall B.Sc.(hons), Dalhousie University, 1993 M.Aq., Simon Fraser University, 1997 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in The Faculty of Graduate Studies (Animal Science) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) March 2012 © Robert Marshall, 2012 Abstract The aim of this thesis was to identify conditions that optimize Panopea generosa broodstock conditioning and larval growth and survival in a hatchery setting. A series of experiments subjected broodstock (adults) to various levels of key factors [i.e. temperature (Ch. 2), salinity (Ch. 3) and nutrition [ration (Ch. 4) and feed type (Ch. 5)]. A larval experiment examined the effects of stocking density and feed level combinations on growth and survival (Ch. 6). Broodstock responses were quantified using gravimetric (condition and gonadosomatic indices) and histological techniques (development classification, volume fractions and oocyte diameter). Survival and spawning rates were also examined. Of the temperatures tested (7, 11, 15 and 19˚C) 11˚C had the highest spawning rates (% individuals) and more oocytes follicle-1, than 15 and 19˚C. At 7˚C gonadosomatic indices were highest but this temperature did not produce spawning clams. Gonads degenerated at 19˚C. Among salinities of 17, 20, 24, and 29 gonad sheath thickness and area occupied by gametes increased at 29 but not at 24. Salinities of 17 and 20 were associated with fungal infection and had high mortality rates after 26 d exposure. With higher ration treatments (up to 7.2 × 109 cells clam-1 d-1 [Isochrysis sp. -

Biogeographic Patterns of the Marine Bivalve Cerastoderma Edule Along European Atlantic Coasts
Biogeographic patterns of the marine bivalve Cerastoderma edule along European Atlantic coasts DISSERTATION Zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Christian-Albrecht-Universität zu Kiel vorgelegt von Manuela Krakau Kiel 2008 Angefertigt an der Wattenmeerstation Sylt Stiftung Alfred-Wegener-Institut für Polar- und Meeresforschung Referent: Prof. Dr. Karsten Reise Koreferent: Prof. Dr. Reinhold Hanel Tag der mündlichen Prüfung: 10. Juli 2008 Zum Druck genehmigt: 10. Juli 2008 CONTENTS SUMMARY ...…...………………………………….………………………… I ZUSAMMENFASSUNG .……………………………………………………... III GENERAL INTRODUCTION .………………………………………………….. 1 CHAPTER 1: Shell forms of the intertidal bivalve Cerastoderma edule L. from Africa to the Arctic .………………………………………… 9 CHAPTER 2: Cockle parasites across biogeographic provinces ..……......… 36 CHAPTER 3: Genetic diversity in high latitudes – an intertidal bivalve contradicts a common pattern ..…………………………… 59 GENERAL DISCUSSION .…………………...……………………………….. 89 REFERENCES …..…………………………………………………………... 95 APPENDIX ………………………………………………………………… X1 ACKNOWLEDGEMENTS /D ANKSAGUNG Biogeographic patterns of the marine bivalve C. edule SUMMARY SUMMARY The cockle Cerastoderma edule is a common bivalve that inhabits the marine soft-bottom intertidal along European shores. This invertebrate plays a key role in coastal food webs of the Northeast Atlantic coasts due of its high abundances. I studied cockles from 19 sites along the distribution range with the aim to describe the variation of geographic population