Traditional Use and Hnrsst Rates of Shellfish Especially Razor Clams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

COMPLETE LIST of MARINE and SHORELINE SPECIES 2012-2016 BIOBLITZ VASHON ISLAND Marine Algae Sponges
COMPLETE LIST OF MARINE AND SHORELINE SPECIES 2012-2016 BIOBLITZ VASHON ISLAND List compiled by: Rayna Holtz, Jeff Adams, Maria Metler Marine algae Number Scientific name Common name Notes BB year Location 1 Laminaria saccharina sugar kelp 2013SH 2 Acrosiphonia sp. green rope 2015 M 3 Alga sp. filamentous brown algae unknown unique 2013 SH 4 Callophyllis spp. beautiful leaf seaweeds 2012 NP 5 Ceramium pacificum hairy pottery seaweed 2015 M 6 Chondracanthus exasperatus turkish towel 2012, 2013, 2014 NP, SH, CH 7 Colpomenia bullosa oyster thief 2012 NP 8 Corallinales unknown sp. crustous coralline 2012 NP 9 Costaria costata seersucker 2012, 2014, 2015 NP, CH, M 10 Cyanoebacteria sp. black slime blue-green algae 2015M 11 Desmarestia ligulata broad acid weed 2012 NP 12 Desmarestia ligulata flattened acid kelp 2015 M 13 Desmerestia aculeata (viridis) witch's hair 2012, 2015, 2016 NP, M, J 14 Endoclaydia muricata algae 2016 J 15 Enteromorpha intestinalis gutweed 2016 J 16 Fucus distichus rockweed 2014, 2016 CH, J 17 Fucus gardneri rockweed 2012, 2015 NP, M 18 Gracilaria/Gracilariopsis red spaghetti 2012, 2014, 2015 NP, CH, M 19 Hildenbrandia sp. rusty rock red algae 2013, 2015 SH, M 20 Laminaria saccharina sugar wrack kelp 2012, 2015 NP, M 21 Laminaria stechelli sugar wrack kelp 2012 NP 22 Mastocarpus papillatus Turkish washcloth 2012, 2013, 2014, 2015 NP, SH, CH, M 23 Mazzaella splendens iridescent seaweed 2012, 2014 NP, CH 24 Nereocystis luetkeana bull kelp 2012, 2014 NP, CH 25 Polysiphonous spp. filamentous red 2015 M 26 Porphyra sp. nori (laver) 2012, 2013, 2015 NP, SH, M 27 Prionitis lyallii broad iodine seaweed 2015 M 28 Saccharina latissima sugar kelp 2012, 2014 NP, CH 29 Sarcodiotheca gaudichaudii sea noodles 2012, 2014, 2015, 2016 NP, CH, M, J 30 Sargassum muticum sargassum 2012, 2014, 2015 NP, CH, M 31 Sparlingia pertusa red eyelet silk 2013SH 32 Ulva intestinalis sea lettuce 2014, 2015, 2016 CH, M, J 33 Ulva lactuca sea lettuce 2012-2016 ALL 34 Ulva linza flat tube sea lettuce 2015 M 35 Ulva sp. -

Paleoenvironmental Interpretation of Late Glacial and Post
PALEOENVIRONMENTAL INTERPRETATION OF LATE GLACIAL AND POST- GLACIAL FOSSIL MARINE MOLLUSCS, EUREKA SOUND, CANADIAN ARCTIC ARCHIPELAGO A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Department of Geography University of Saskatchewan Saskatoon By Shanshan Cai © Copyright Shanshan Cai, April 2006. All rights reserved. i PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Head of the Department of Geography University of Saskatchewan Saskatoon, Saskatchewan S7N 5A5 i ABSTRACT A total of 5065 specimens (5018 valves of bivalve and 47 gastropod shells) have been identified and classified into 27 species from 55 samples collected from raised glaciomarine and estuarine sediments, and glacial tills. -

Performance of the New England Hydraulic Dredge for the Harvest Of'stimpson~Surf Clams
PERFORMANCE OF THE NEW ENGLAND HYDRAULIC DREDGE FOR THE HARVEST OF'STIMPSON~SURF CLAMS Inv d Experimental Biology Division*. Deparhnent of Fisheries and Oceans Canada Maurice Lamontagne Instihite 850 route de la Mer f O00 Mont-Joli (~uébec) G5H 324 Canadian Industry Report of isheries and Aquatic Sciences 235 ? nadian Industry Report of Fisheries and Aquatic Sciences canaain the ~e?iaIts:Bf ramh and develotrment iradustq fof MkF irnmedia~elar Iutuw1appdkashn. They are da.r.ected pfimarbly tmard indiziduak .h $ha primary and secondary çccrors of the fishing and marine mduaries. ?i,o ratlrMhn k phced on sulbjmt mcta and the series reflects the broad inoemrs and p~2ek5&oh Department d Fisher& and Oceans. aamely. fishiesad aqwticsc+awe~,. .' 1t@u~rr5reprts m. be cimed & full pnsblicatbns. The cornkt citatian aowars . abri the abatracr of each report. Eacb rekl is a&rrxad in Awair %ie.nr& ifid . technical publicatio . Sumber;l-9li e lndustrial Devel- opsnent BranCh. Technical Reports of th. lndiausrrkf ikxelo~mentÉranch. a& - ,Tecknical Reports of the &-sk*an's Servk Branch. kumkrs 62- I tO Giztaisseed as . Dapan~enzof Fishsies and the Em-i~onment.Fisher& and Màrine Service fndustry Reports. The current ysies name kas chmgel ~ithreport number 111. lndwsrr! reports are produc& rqiortdk bur are numkred nationrtlty. Requests .for indi\ idual rqporfs w il1 be fifkd by the issuing~rqblishmentl~tcdon the front cûvw and title page. Out-of-stwk feports-will k svpplied for fw? b'J; commacia~agents. Les rapporis 4 1'indlasrrk am t knmf k résultats des acîivitis de mher&c et de diveloppern-enl qui peuvent &se utiles a I'industrie gour des applications immédiates ou fatures. -

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -

Circular 162. the Soft-Shell Clam
HE OFT iHELL 'LAM UNITED STATES DEPARTMENT 8F THE INTERIOR FISH AND WILDLIFE SERVICE BUREAU OF COMMERCIAL FISHERIES Circular 162 CONTENTS Page 1 Intr oduc tion. • • • . 2 Natural history • . ~ . 2 Distribution. 3 Taxonomy. · . Anatomy. • • • •• . · . 3 4 Life cycle. • • • • • . • •• Predators, diseases, and parasites . 7 Fishery - methods and management .•••.••• • 9 New England area. • • • • • . • • • • • . .. 9 Chesapeake area . ........... 1 1 Special problem s of paralytic shellfish poisoning and pollution . .............. 13 Summary . .... 14 Acknowledgment s •••• · . 14 Selected bibliography •••••• 15 ABSTRACT Describes the soft- shell cla.1n industry of the Atlantic coast; reviewing past and present economic importance, fishery methods, fishery management programs, and special problems associated with shellfish culture and marketing. Provides a summary of soft- shell clam natural history in- eluding distribution, taxonomy, anatomy, and life cycle. l THE SOFT -SHELL CLAM by Robert W. Hanks Bureau of Commercial Fisheries Biological Labor ator y U.S. Fish and Wildlife Serv i c e Boothbay Harbor, Maine INTRODUCTION for s a lted c l a m s used as bait by cod fishermen on t he G r and Bank. For the next The soft-shell clam, Myaarenaria L., has 2 5 years this was the most important outlet played an important role in the history and for cla ms a nd was a sour ce of wealth to economy of the eastern coast of our country . some coastal communities . Many of the Long before the first explorers reached digg ers e a rne d up t o $10 per day in this our shores, clams were important in the busin ess during October to March, when diet of some American Indian tribes. -
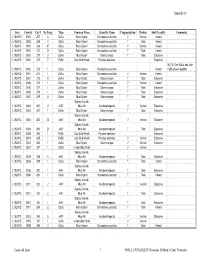
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

Download Download
Appendix C: An Analysis of Three Shellfish Assemblages from Tsʼishaa, Site DfSi-16 (204T), Benson Island, Pacific Rim National Park Reserve of Canada by Ian D. Sumpter Cultural Resource Services, Western Canada Service Centre, Parks Canada Agency, Victoria, B.C. Introduction column sampling, plus a second shell data collect- ing method, hand-collection/screen sampling, were This report describes and analyzes marine shellfish used to recover seven shellfish data sets for investi- recovered from three archaeological excavation gating the siteʼs invertebrate materials. The analysis units at the Tseshaht village of Tsʼishaa (DfSi-16). reported here focuses on three column assemblages The mollusc materials were collected from two collected by the researcher during the 1999 (Unit different areas investigated in 1999 and 2001. The S14–16/W25–27) and 2001 (Units S56–57/W50– source areas are located within the village proper 52, S62–64/W62–64) excavations only. and on an elevated landform positioned behind the village. The two areas contain stratified cultural Procedures and Methods of Quantification and deposits dating to the late and middle Holocene Identification periods, respectively. With an emphasis on mollusc species identifica- The primary purpose of collecting and examining tion and quantification, this preliminary analysis the Tsʼishaa shellfish remains was to sample, iden- examines discarded shellfood remains that were tify, and quantify the marine invertebrate species collected and processed by the site occupants for each major stratigraphic layer. Sets of quantita- for approximately 5,000 years. The data, when tive information were compiled through out the reviewed together with the recovered vertebrate analysis in order to accomplish these objectives. -

THE NAUTILUS [Vol
2 2 THE NAUTILUS [Vol. 69 (1) separated from each other by five centimeters. The snail was expanded with its head oriented away from the clam. When placed on the sand, the clam showed no activity during the next 30 minutes after which observations were discontinued. All but the lower (anterior) end of the body of the clam was covered by an envelope of slime secreted by the foot of the snail. It seems clear that P. duplicatus capturesEnsis directus by approaching it below the surface of the substratum and by ir ritating the lower portion so that it retreats upward. The snail then coats the razor clam with an envelope of slime which ap pears to have anesthetic properties. Successful capture proba bly depends on the ability of the snail to maintain contact with its prey until anesthesia takes place. ON THE OCCURRENCE OF THE NUDIBRANCH ALDERIA MODESTA (LOVÉN, 1844) ON THE CENTRAL CALIFORNIAN COAST By CADET HAND and JOAN STEINBERG Department of Zoology, University of California, Berkeley Alderia modesta (Loven, 1844) has long been known from the coasts of northern Europe. It has been recorded from as far north as the Trondheim Fjord in Norway (Norman, 1893), south to Skibbereen in Ireland (Allman, 1845) and on the French coast (Gollien, 1929). Therefore, it has been of con siderable interest to us to find well-established populations of an Alderia in two localities on the central Californian coast. Through the kindness of Monsieur G. Van Put of the Royal Institute of Natural Sciences of Belgium in Brussels and Dr. -

Giant Pacific Octopus (Enteroctopus Dofleini) Care Manual
Giant Pacific Octopus Insert Photo within this space (Enteroctopus dofleini) Care Manual CREATED BY AZA Aquatic Invertebrate Taxonomic Advisory Group IN ASSOCIATION WITH AZA Animal Welfare Committee Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Aquatic Invertebrate Taxon Advisory Group (AITAG) (2014). Giant Pacific Octopus (Enteroctopus dofleini) Care Manual. Association of Zoos and Aquariums, Silver Spring, MD. Original Completion Date: September 2014 Dedication: This work is dedicated to the memory of Roland C. Anderson, who passed away suddenly before its completion. No one person is more responsible for advancing and elevating the state of husbandry of this species, and we hope his lifelong body of work will inspire the next generation of aquarists towards the same ideals. Authors and Significant Contributors: Barrett L. Christie, The Dallas Zoo and Children’s Aquarium at Fair Park, AITAG Steering Committee Alan Peters, Smithsonian Institution, National Zoological Park, AITAG Steering Committee Gregory J. Barord, City University of New York, AITAG Advisor Mark J. Rehling, Cleveland Metroparks Zoo Roland C. Anderson, PhD Reviewers: Mike Brittsan, Columbus Zoo and Aquarium Paula Carlson, Dallas World Aquarium Marie Collins, Sea Life Aquarium Carlsbad David DeNardo, New York Aquarium Joshua Frey Sr., Downtown Aquarium Houston Jay Hemdal, Toledo -

Spisula Solidissima) Using a Spatially Northeastern Continental Shelf of the United States
300 Abstract—The commercially valu- able Atlantic surfclam (Spisula so- Management strategy evaluation for the Atlantic lidissima) is harvested along the surfclam (Spisula solidissima) using a spatially northeastern continental shelf of the United States. Its range has con- explicit, vessel-based fisheries model tracted and shifted north, driven by warmer bottom water temperatures. 1 Declining landings per unit of effort Kelsey M. Kuykendall (contact author) (LPUE) in the Mid-Atlantic Bight Eric N. Powell1 (MAB) is one result. Declining stock John M. Klinck2 abundance and LPUE suggest that 1 overfishing may be occurring off Paula T. Moreno New Jersey. A management strategy Robert T. Leaf1 evaluation (MSE) for the Atlantic surfclam is implemented to evalu- Email address for contact author: [email protected] ate rotating closures to enhance At- lantic surfclam productivity and in- 1 Gulf Coast Research Laboratory crease fishery viability in the MAB. The University of Southern Mississippi Active agents of the MSE model 703 East Beach Drive are individual fishing vessels with Ocean Springs, Mississippi 39564 performance and quota constraints 2 Center for Coastal Physical Oceanography influenced by captains’ behavior Department of Ocean, Earth, and Atmospheric Sciences over a spatially varying population. 4111 Monarch Way, 3rd Floor Management alternatives include Old Dominion University 2 rules regarding closure locations Norfolk, Virginia 23529 and 3 rules regarding closure du- rations. Simulations showed that stock biomass increased, up to 17%, under most alternative strategies in relation to estimated stock biomass under present-day management, and The Atlantic surfclam (Spisula solid- ally not found where average bottom LPUE increased under most alterna- issima) is an economically valuable temperatures exceed 25°C (Cargnelli tive strategies, by up to 21%. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber .........................................................................