Shell Shape Variation in Populations of Common Cockle Anadara Oceanica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Invertebrate Animals (Metazoa: Invertebrata) of the Atanasovsko Lake, Bulgaria
Historia naturalis bulgarica, 22: 45-71, 2015 Invertebrate Animals (Metazoa: Invertebrata) of the Atanasovsko Lake, Bulgaria Zdravko Hubenov, Lyubomir Kenderov, Ivan Pandourski Abstract: The role of the Atanasovsko Lake for storage and protection of the specific faunistic diversity, characteristic of the hyper-saline lakes of the Bulgarian seaside is presented. The fauna of the lake and surrounding waters is reviewed, the taxonomic diversity and some zoogeographical and ecological features of the invertebrates are analyzed. The lake system includes from freshwater to hyper-saline basins with fast changing environment. A total of 6 types, 10 classes, 35 orders, 82 families and 157 species are known from the Atanasovsko Lake and the surrounding basins. They include 56 species (35.7%) marine and marine-brackish forms and 101 species (64.3%) brackish-freshwater, freshwater and terrestrial forms, connected with water. For the first time, 23 species in this study are established (12 marine, 1 brackish and 10 freshwater). The marine and marine- brackish species have 4 types of ranges – Cosmopolitan, Atlantic-Indian, Atlantic-Pacific and Atlantic. The Atlantic (66.1%) and Cosmopolitan (23.2%) ranges that include 80% of the species, predominate. Most of the fauna (over 60%) has an Atlantic-Mediterranean origin and represents an impoverished Atlantic-Mediterranean fauna. The freshwater-brackish, freshwater and terrestrial forms, connected with water, that have been established from the Atanasovsko Lake, have 2 main types of ranges – species, distributed in the Palaearctic and beyond it and species, distributed only in the Palaearctic. The representatives of the first type (52.4%) predomi- nate. They are related to the typical marine coastal habitats, optimal for the development of certain species. -

Anadara Kagoshimensis (Mollusca: Bivalvia: Arcidae)
Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net http://dx.doi.org/10.12681/mms.2076 Anadara kagoshimensis (Mollusca: Bivalvia: Arcidae) in the Adriatic Sea: morphological analysis, molecular taxonomy, spatial distribution, and prediction PIERLUIGI STRAFELLA1, ALICE FERRARI2, GIANNA FABI1, VERA SALVALAGGIO1, ELISA PUNZO1, CLARA CUICCHI1, ANGELA SANTELLI1, ALESSIA CARIANI2, FAUSTO TINTI2, ANNA NORA TASSETTI1 and GIUSEPPE SCARCELLA1 1 Istituto di Scienze Marine (ISMAR), Consiglio Nazionale delle Ricerche (CNR), L.go Fiera della Pesca, 2, 60125 Ancona, Italy 2 Department of Biological, Geological & Environmental Sciences (BiGeA), University of Bologna, Via Selmi, 3, 40126, Bologna, Italy Corresponding author: [email protected] Handling Editor: Fabio Crocetta Received: 11 October 2016; Accepted: 3 August 2017; Published on line: 7 December 2017 Abstract Morphological analysis, molecular characterization, and a study of the distribution and density of Anadara kagoshimensis (Tokunaga, 1906) specimens collected in the Adriatic Sea were carried out using materials and data collected in the course of 329 bottom trawl hauls conducted in five yearly surveys, from 2010 to 2014. Morphological and molecular analysis allowed clarifying the confused taxonomy of the largest alien ark clam species invading Italian waters and the Mediterranean Sea. Analysis of the distribution and density data demonstrated that, along the Italian coast, A. kagoshimensis is mostly found at depths of 8 to 50 m, with a catch frequency of more than 98% in the hauls involving silty-clay sediment at a depth of 8-30 m. The hotspot map clearly shows a reduction in the distribution area of the species from 2010 to 2012. -

MOLLUSCS Species Names – for Consultation 1
MOLLUSCS species names – for consultation English name ‘Standard’ Gaelic name Gen Scientific name Notes Neologisms in italics der MOLLUSC moileasg m MOLLUSCS moileasgan SEASHELL slige mhara f SEASHELLS sligean mara SHELLFISH (singular) maorach m SHELLFISH (plural) maoraich UNIVALVE SHELLFISH aon-mhogalach m (singular) UNIVALVE SHELLFISH aon-mhogalaich (plural) BIVALVE SHELLFISH dà-mhogalach m (singular) BIVALVE SHELLFISH dà-mhogalaich (plural) LIMPET (general) bàirneach f LIMPETS bàirnich common limpet bàirneach chumanta f Patella vulgata ‘common limpet’ slit limpet bàirneach eagach f Emarginula fissura ‘notched limpet’ keyhole limpet bàirneach thollta f Diodora graeca ‘holed limpet’ china limpet bàirneach dhromanach f Patella ulyssiponensis ‘ridged limpet’ blue-rayed limpet copan Moire m Patella pellucida ‘The Virgin Mary’s cup’ tortoiseshell limpet bàirneach riabhach f Testudinalia ‘brindled limpet’ testudinalis white tortoiseshell bàirneach bhàn f Tectura virginea ‘fair limpet’ limpet TOP SHELL brùiteag f TOP SHELLS brùiteagan f painted top brùiteag dhotamain f Calliostoma ‘spinning top shell’ zizyphinum turban top brùiteag thurbain f Gibbula magus ‘turban top shell’ grey top brùiteag liath f Gibbula cineraria ‘grey top shell’ flat top brùiteag thollta f Gibbula umbilicalis ‘holed top shell’ pheasant shell slige easaig f Tricolia pullus ‘pheasant shell’ WINKLE (general) faochag f WINKLES faochagan f banded chink shell faochag chlaiseach bhannach f Lacuna vincta ‘banded grooved winkle’ common winkle faochag chumanta f Littorina littorea ‘common winkle’ rough winkle (group) faochag gharbh f Littorina spp. ‘rough winkle’ small winkle faochag bheag f Melarhaphe neritoides ‘small winkle’ flat winkle (2 species) faochag rèidh f Littorina mariae & L. ‘flat winkle’ 1 MOLLUSCS species names – for consultation littoralis mudsnail (group) seilcheag làthaich f Fam. -

Physiological Responses to Ocean Acidification and Warming
Marine Environmental Research 130 (2017) 38e47 Contents lists available at ScienceDirect Marine Environmental Research journal homepage: www.elsevier.com/locate/marenvrev Physiological responses to ocean acidification and warming synergistically reduce condition of the common cockle Cerastoderma edule * E.Z. Ong a, b, , M. Briffa b, T. Moens a, C. Van Colen a a Ghent University, Biology Department, Marine Biology Research Group, Krijgslaan 281eS8, B 9000 Ghent, Belgium b Marine Biology & Ecology Research Centre, Plymouth University, Plymouth PL4 8AA, UK article info abstract Article history: The combined effect of ocean acidification and warming on the common cockle Cerastoderma edule was Received 4 February 2017 investigated in a fully crossed laboratory experiment. Survival of the examined adult organisms remained Received in revised form high and was not affected by elevated temperature (þ3 C) or lowered pH (À0.3 units). However, the 6 June 2017 morphometric condition index of the cockles incubated under high pCO conditions (i.e. combined Accepted 3 July 2017 2 warming and acidification) was significantly reduced after six weeks of incubation. Respiration rates Available online 5 July 2017 increased significantly under low pH, with highest rates measured under combined warm and low pH conditions. Calcification decreased significantly under low pH while clearance rates increased signifi- Keywords: Future ocean cantly under warm conditions and were generally lower in low pH treatments. The observed physio- fi Ocean acidification logical responses suggest that the reduced food intake under hypercapnia is insuf cient to support the Ocean warming higher energy requirements to compensate for the higher costs for basal maintenance and growth in Cerastoderma edule future high pCO2 waters. -

Alien Species in the Mediterranean Sea by 2010
Mediterranean Marine Science Review Article Indexed in WoS (Web of Science, ISI Thomson) The journal is available on line at http://www.medit-mar-sc.net Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution A. ZENETOS 1, S. GOFAS 2, M. VERLAQUE 3, M.E. INAR 4, J.E. GARCI’A RASO 5, C.N. BIANCHI 6, C. MORRI 6, E. AZZURRO 7, M. BILECENOGLU 8, C. FROGLIA 9, I. SIOKOU 10 , D. VIOLANTI 11 , A. SFRISO 12 , G. SAN MART N 13 , A. GIANGRANDE 14 , T. KATA AN 4, E. BALLESTEROS 15 , A. RAMOS-ESPLA ’16 , F. MASTROTOTARO 17 , O. OCA A 18 , A. ZINGONE 19 , M.C. GAMBI 19 and N. STREFTARIS 10 1 Institute of Marine Biological Resources, Hellenic Centre for Marine Research, P.O. Box 712, 19013 Anavissos, Hellas 2 Departamento de Biologia Animal, Facultad de Ciencias, Universidad de Ma ’laga, E-29071 Ma ’laga, Spain 3 UMR 6540, DIMAR, COM, CNRS, Université de la Méditerranée, France 4 Ege University, Faculty of Fisheries, Department of Hydrobiology, 35100 Bornova, Izmir, Turkey 5 Departamento de Biologia Animal, Facultad de Ciencias, Universidad de Ma ’laga, E-29071 Ma ’laga, Spain 6 DipTeRis (Dipartimento per lo studio del Territorio e della sue Risorse), University of Genoa, Corso Europa 26, 16132 Genova, Italy 7 Institut de Ciències del Mar (CSIC) Passeig Mar tim de la Barceloneta, 37-49, E-08003 Barcelona, Spain 8 Adnan Menderes University, Faculty of Arts & Sciences, Department of Biology, 09010 Aydin, Turkey 9 c\o CNR-ISMAR, Sede Ancona, Largo Fiera della Pesca, 60125 Ancona, Italy 10 Institute of Oceanography, Hellenic Centre for Marine Research, P.O. -

Mercury Concentration in Bivalve Molluscs
Bull Vet Inst Pulawy 59, 357-360, 2015 DOI:10.1515/bvip-2015-0053 Mercury concentration in bivalve molluscs Józef Szkoda1, Maciej Durkalec1, Agnieszka Nawrocka1, Mirosław Michalski2 1Department of Pharmacology and Toxicology, 2Department of Hygiene of Animal Origin, National Veterinary Research Institute, 24-100 Pulawy, Poland [email protected] Received: April 23, 2015 Accepted: September 4, 2015 Abstract A total of 85 mussel samples of eight species were examined. Analysis of mercury in the freeze-dried samples was carried out by atomic absorption spectrometry method using direct mercury analyser AMA 254. The analytical procedure for determination of mercury was covered by the quality assurance programme of research and participation in national and international proficiency tests. Concentrations of total mercury in all investigated samples were found to be generally low, in the range of 0.033-0.577 mg/kg of dry weight and of 0.003-0.045 mg/kg of wet weight. The results indicate that obtained levels of mercury in bivalve molluscs are not likely to pose a risk to the health of consumers. Keywords: mercury, bivalve molluscs, bioaccumulation, food safety. Introduction which may pose a threat to the health of consumers (7, 13). In Poland, the interest in marine food, or seafood, The rules for the marketing of live bivalve has increased in the recent years. Crustaceans, mostly molluscs for direct consumption and also for shrimps, squids, and lobsters, are frequently chosen processing are strictly defined in EU law regulating by consumers, but less popular oysters, cockles, all aspects of production, including environmental mussels, and clams which belong to bivalve molluscs conditions, farming, cleaning, transport, sale, are also purchased. -
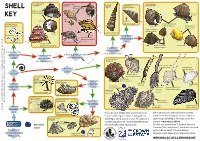
Shell Whelk Dog Whelk Turret It Could Be a Periwinkle Shell (Nucella Lapillus) Shell Spire Shell Thick Top Shell (Osilinus Lineatus) Dark Stripes Key on Body
It could be a type of It could be a type of It could be a It could be a type of topshell whelk Dog whelk turret It could be a periwinkle Shell (Nucella lapillus) shell spire shell Thick top shell (Osilinus lineatus) Dark stripes Key on body Egg Underside capsules Actual size It could be a type of (Hydrobia sp) Common periwinkle spiral worm White ‘Colar’ (Littorina littorea) Flat periwinkle (Littorinasp) Yes Roughly ‘ribbed’ shell. Very high up shore ‘Tooth inside (Turitella communis) opening (Spirorbis sp) Does it have 6 Common whelk No (Buccinum undatum) Yes or more whorls Brown, speckled Netted dog whelk body (twists)? Painted topshell (Nassarius reticulatus) (Calliostoma zizyphinum) No Rough periwinkle Flattened spire Yes Is it long, thin (Littorina saxatilis) Yes Yes and cone shaped Is it permanently No like a unicorn’s horn? attached to Is there a groove or teeth No Is there mother No a surface? in the shell opening? of pearl inside It could be a type of the shell opening? bivalve Yes Yes Common otter-shell (Lutraria lutraria) Bean-like tellin No Is the shell in (Fabulina fabula) Is it 2 parts? spiraled? Common cockle (Cerastoderma edule) It could be a Flat, rounded No sand No Great scallop mason It could be a Is the shell a (Pecten maximus) shell Razor shell worm keel worm Wedge-shaped Is the case dome or (Ensis sp) No Pacific oyster shell made from Yes cone shape? (Crassostrea gigas) Shell can be Peppery furrow shell very large (Scrobicularia plana) sand grains? Elongated and and doesn’t (Lanice conchilega) deep-bodied fully close with large ‘frills’ No (Pomatoceros sp) Yes It could be a type of sea urchin It could be a type of An acorn Native oyster Empty barnacle barnacle Does it have that may be found in estuaries and shores in the UK. -

Impact of Trematode Parasitism on the Fauna of a North Sea Tidal Flat
HELGOI~NDER MEERESUNTERSUCHUNGEN Helgol~nder Meeresunters. 37, 185-199 (1984) Impact of trematode parasitism on the fauna of a North Sea tidal flat G. Lauckner Biologische Anstalt Helgoland (Litoralstation]; D-2282 List/Sylt, Federal Republic of Germany ABSTRACT: The impact of larval trematodes on the fauna of a North Sea tidal flat is considered at the individual and at the population level, depicting the digenean parasites of the common periwinkle, Littorina littorea, and their life cycles, as an example. On the German North Sea coast, L. fittorea is first intermediate host for 6 larval trematodes representing 6 digenean families - Cryptocotyle lingua (Heterophyidae), Himasthla elongata (Echinostomatidae), Renicola roscovita (Renicolidae), Microphallus pygmaeus (Microphallidae), Podocotyle atomon (Opecoelidae} and Cercaria lebouri (Notocotylidae). All except P. atomon utilize shore birds as final hosts; adult P. atomon parasitize in the intestine of teleosts, mainly pleuronectid flatfish. Second intermediate hosts of C. lingua are various species of fish; the cercariae of H. elongata encyst in molluscs and polychaetes, those of R. roscovita in molluscs; Iv[. pygmaeus has an abbreviated life cycle; C. lebouri encysts free on solid surfaces; and the fish trematode P. atomon utilizes benthic crustaceans, mainly amphipods, as second intermediate hosts. On the tidal flats of the K6nigshafen (Sylt), up to 77 % of the periwinkles have been found to be infested by larval trematodes. Maximum infestations in individual samples were 23 % for C. lingua, 47 % for H. etongata and 44 To for R. roscovita. The digeneans cause complete 'parasitic castration' of their carriers and hence exclude a considerable proportion of the snails from the breeding population. -

Solent Bivalve Survey 2019 Report
Solent Bivalve Stock Survey Spring 2019 This report has been produced by the Southern Inshore Fisheries and Conservation Authority. A copy of this report is available on our website at www.southern-ifca.gov.uk or from the Southern IFCA Office at: Unit 3, Holes Bay Park Sterte Road West Poole Dorset BH15 2AA 01202 721373 [email protected] !1 Contents 1. Introduction 3 1.1. The Fishery 3 1.2. The Solent 4 1.3. Current Management 5 2. Methodology 5 2.1. The Survey 5 2.2. Equipment 6 2.3. Data Analysis 6 3. Results 6 3.1. Southampton Water 7 3.2. Portsmouth Harbour 9 3.3. Langstone Harbour 11 4. Discussion 13 4.1. Southampton Water 13 4.2. Portsmouth Harbour 14 4.3. Langstone Harbour 15 4.4. Cockle 16 4.5. American Hard-Shelled clam 17 5. References 17 6. Annex 18 6.1. Annex 1 18 6.2. Annex 2 21 !2 1. Introduction The following report details the bivalve 1 surveys carried out in Southampton Water, Portsmouth Harbour and Langstone Harbour during March - April 2018, October 2018, and March 2019. The report will assess the distribution and abundance of clam and cockle species over time to evaluate the population health and stability of commercially important species for the dredge fishery. In addition, the outcomes from the survey will provide data which can be used as a baseline on which to monitor 2 future trends and potential changes to populations which will feed into the development and monitoring of local management strategies. -

Anadara Inaequivalvis) Ecological Risk Screening Summary
Arc clam (Anadara inaequivalvis) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, Web Version – 11/27/2017 Photo: Andrew Butko. Licensed under Creative Commons BY-SA 3.0 Unported. Available: http://eol.org/data_objects/27712609. 1 Native Range and Status in the United States Native Range From Sahin et al. (2009): “[…] blood-cockle [A. inaequivalvis] are Indo-Pacific origin.” From Lutaenko (1993): “Distribution: India, Burma, Thailand, Malaya, Indonesia, North Australia, Philippines, Japan, China […]” Status in the United States No records of Anadara inaequivalvis in the United States were found. 1 Means of Introductions in the United States No records of Anadara inaequivalvis in the United States were found. Remarks Some records refer to Anadara inaequivalvis using the synonym Scapharca inaequivalvis. Information searches were performed using both names. From Gofas (2004): “Anadara kagoshimensis (Tokunaga, 1906) is the valid name for an invasive species in the Mediterranean and Black Sea. It has earlier been misidentified and reported under the names Scapharca cornea and Anadara inaequivalvis. Anadara cornea (Reeve, 1844) and Anadara inaequivalvis (Bruguière, 1789) are two valid species that do not occur in the Mediterranean and Black Sea, neither as a native nor as an introduced species.” From Zenetos et al. (2010): “The Adriatic holds only 27 alien species (15 established, nine casual and three cryptogenic) but the striking characteristic is the high proportion of them which have become invasive. Together with the Levantine basin, the Adriatic may be the part of the Mediterranean which has been most transformed by the onset of alien species. The most invasive species include Anadara kagoshimensis (formerly known [identified] as A. -

BROODSTOCK CONDITIONING and LARVAL REARING of the GEODUCK CLAM (Panopea Generosa GOULD, 1850)
BROODSTOCK CONDITIONING AND LARVAL REARING OF THE GEODUCK CLAM (Panopea generosa GOULD, 1850) by Robert Marshall B.Sc.(hons), Dalhousie University, 1993 M.Aq., Simon Fraser University, 1997 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in The Faculty of Graduate Studies (Animal Science) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) March 2012 © Robert Marshall, 2012 Abstract The aim of this thesis was to identify conditions that optimize Panopea generosa broodstock conditioning and larval growth and survival in a hatchery setting. A series of experiments subjected broodstock (adults) to various levels of key factors [i.e. temperature (Ch. 2), salinity (Ch. 3) and nutrition [ration (Ch. 4) and feed type (Ch. 5)]. A larval experiment examined the effects of stocking density and feed level combinations on growth and survival (Ch. 6). Broodstock responses were quantified using gravimetric (condition and gonadosomatic indices) and histological techniques (development classification, volume fractions and oocyte diameter). Survival and spawning rates were also examined. Of the temperatures tested (7, 11, 15 and 19˚C) 11˚C had the highest spawning rates (% individuals) and more oocytes follicle-1, than 15 and 19˚C. At 7˚C gonadosomatic indices were highest but this temperature did not produce spawning clams. Gonads degenerated at 19˚C. Among salinities of 17, 20, 24, and 29 gonad sheath thickness and area occupied by gametes increased at 29 but not at 24. Salinities of 17 and 20 were associated with fungal infection and had high mortality rates after 26 d exposure. With higher ration treatments (up to 7.2 × 109 cells clam-1 d-1 [Isochrysis sp. -

Biogeographic Patterns of the Marine Bivalve Cerastoderma Edule Along European Atlantic Coasts
Biogeographic patterns of the marine bivalve Cerastoderma edule along European Atlantic coasts DISSERTATION Zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Christian-Albrecht-Universität zu Kiel vorgelegt von Manuela Krakau Kiel 2008 Angefertigt an der Wattenmeerstation Sylt Stiftung Alfred-Wegener-Institut für Polar- und Meeresforschung Referent: Prof. Dr. Karsten Reise Koreferent: Prof. Dr. Reinhold Hanel Tag der mündlichen Prüfung: 10. Juli 2008 Zum Druck genehmigt: 10. Juli 2008 CONTENTS SUMMARY ...…...………………………………….………………………… I ZUSAMMENFASSUNG .……………………………………………………... III GENERAL INTRODUCTION .………………………………………………….. 1 CHAPTER 1: Shell forms of the intertidal bivalve Cerastoderma edule L. from Africa to the Arctic .………………………………………… 9 CHAPTER 2: Cockle parasites across biogeographic provinces ..……......… 36 CHAPTER 3: Genetic diversity in high latitudes – an intertidal bivalve contradicts a common pattern ..…………………………… 59 GENERAL DISCUSSION .…………………...……………………………….. 89 REFERENCES …..…………………………………………………………... 95 APPENDIX ………………………………………………………………… X1 ACKNOWLEDGEMENTS /D ANKSAGUNG Biogeographic patterns of the marine bivalve C. edule SUMMARY SUMMARY The cockle Cerastoderma edule is a common bivalve that inhabits the marine soft-bottom intertidal along European shores. This invertebrate plays a key role in coastal food webs of the Northeast Atlantic coasts due of its high abundances. I studied cockles from 19 sites along the distribution range with the aim to describe the variation of geographic population