Differential Transfer Relations of Physical Flux Density Between
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

International System of Units (SI)
International System of Units (SI) National Institute of Standards and Technology (www.nist.gov) Multiplication Prefix Symbol factor 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 * hecto h 101 * deka da 10-1 * deci d 10-2 * centi c 10-3 milli m 10-6 micro µ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a * Prefixes of 100, 10, 0.1, and 0.01 are not formal SI units From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Seven basic SI units Physical quantity Unit Symbol Length meter m Mass kilogram kg Time second s Electrical current ampere A Thermodynamic temperature kelvin K Amount of substance mole mol Luminous intensity candela cd Supplementary SI units Physical quantity Unit Symbol Plane angle radian rad Solid angle steradian sr From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Derived SI units with special names Physical quantity Unit Symbol Derivation Absorbed dose gray Gy J kg-1 Capacitance farad F A s V-1 Conductance siemens S A V-1 Disintegration rate becquerel Bq l s-1 Electrical charge coulomb C A s Electrical potential volt V W A-1 Energy joule J N m Force newton N kg m s-2 Illumination lux lx lm m-2 Inductance henry H V s A-1 Luminous flux lumen lm cd sr Magnetic flux weber Wb V s Magnetic flux density tesla T Wb m-2 Pressure pascal Pa N m-2 Power watt W J s-1 Resistance ohm Ω V A-1 Volume liter L dm3 From: Downs, R.J. -
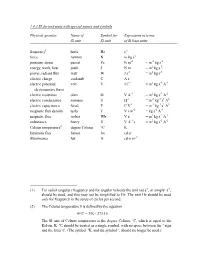
1.4.3 SI Derived Units with Special Names and Symbols
1.4.3 SI derived units with special names and symbols Physical quantity Name of Symbol for Expression in terms SI unit SI unit of SI base units frequency1 hertz Hz s-1 force newton N m kg s-2 pressure, stress pascal Pa N m-2 = m-1 kg s-2 energy, work, heat joule J N m = m2 kg s-2 power, radiant flux watt W J s-1 = m2 kg s-3 electric charge coulomb C A s electric potential, volt V J C-1 = m2 kg s-3 A-1 electromotive force electric resistance ohm Ω V A-1 = m2 kg s-3 A-2 electric conductance siemens S Ω-1 = m-2 kg-1 s3 A2 electric capacitance farad F C V-1 = m-2 kg-1 s4 A2 magnetic flux density tesla T V s m-2 = kg s-2 A-1 magnetic flux weber Wb V s = m2 kg s-2 A-1 inductance henry H V A-1 s = m2 kg s-2 A-2 Celsius temperature2 degree Celsius °C K luminous flux lumen lm cd sr illuminance lux lx cd sr m-2 (1) For radial (angular) frequency and for angular velocity the unit rad s-1, or simply s-1, should be used, and this may not be simplified to Hz. The unit Hz should be used only for frequency in the sense of cycles per second. (2) The Celsius temperature θ is defined by the equation θ/°C = T/K - 273.15 The SI unit of Celsius temperature is the degree Celsius, °C, which is equal to the Kelvin, K. -

EL Light Output Definition.Pdf
GWENT GROUP ADVANCED MATERIAL SYSTEMS Luminance The candela per square metre (cd/m²) is the SI unit of luminance; nit is a non-SI name also used for this unit. It is often used to quote the brightness of computer displays, which typically have luminance’s of 50 to 300 nits (the sRGB spec for monitor’s targets 80 nits). Modern flat-panel (LCD and plasma) displays often exceed 300 cd/m² or 300 nits. The term is believed to come from the Latin "nitere" = to shine. Candela per square metre 1 Kilocandela per square metre 10-3 Candela per square centimetre 10-4 Candela per square foot 0,09 Foot-lambert 0,29 Lambert 3,14×10-4 Nit 1 Stilb 10-4 Luminance is a photometric measure of the density of luminous intensity in a given direction. It describes the amount of light that passes through or is emitted from a particular area, and falls within a given solid angle. The SI unit for luminance is candela per square metre (cd/m2). The CGS unit of luminance is the stilb, which is equal to one candela per square centimetre or 10 kcd/m2 Illuminance The lux (symbol: lx) is the SI unit of illuminance and luminous emittance. It is used in photometry as a measure of the intensity of light, with wavelengths weighted according to the luminosity function, a standardized model of human brightness perception. In English, "lux" is used in both singular and plural. Microlux 1000000 Millilux 1000 Lux 1 Kilolux 10-3 Lumen per square metre 1 Lumen per square centimetre 10-4 Foot-candle 0,09 Phot 10-4 Nox 1000 In photometry, illuminance is the total luminous flux incident on a surface, per unit area. -

Dimensional Analysis and Modeling
cen72367_ch07.qxd 10/29/04 2:27 PM Page 269 CHAPTER DIMENSIONAL ANALYSIS 7 AND MODELING n this chapter, we first review the concepts of dimensions and units. We then review the fundamental principle of dimensional homogeneity, and OBJECTIVES Ishow how it is applied to equations in order to nondimensionalize them When you finish reading this chapter, you and to identify dimensionless groups. We discuss the concept of similarity should be able to between a model and a prototype. We also describe a powerful tool for engi- ■ Develop a better understanding neers and scientists called dimensional analysis, in which the combination of dimensions, units, and of dimensional variables, nondimensional variables, and dimensional con- dimensional homogeneity of equations stants into nondimensional parameters reduces the number of necessary ■ Understand the numerous independent parameters in a problem. We present a step-by-step method for benefits of dimensional analysis obtaining these nondimensional parameters, called the method of repeating ■ Know how to use the method of variables, which is based solely on the dimensions of the variables and con- repeating variables to identify stants. Finally, we apply this technique to several practical problems to illus- nondimensional parameters trate both its utility and its limitations. ■ Understand the concept of dynamic similarity and how to apply it to experimental modeling 269 cen72367_ch07.qxd 10/29/04 2:27 PM Page 270 270 FLUID MECHANICS Length 7–1 ■ DIMENSIONS AND UNITS 3.2 cm A dimension is a measure of a physical quantity (without numerical val- ues), while a unit is a way to assign a number to that dimension. -
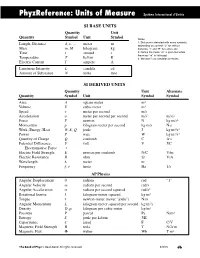
Units & Quantities Reference
PhyzReference: Units of Measure Systéme International d‘Unitès SI BASE UNITS Quantity Unit Quantity Symbol Unit Symbol Notes 1. Distance is denoted with many symbols, Length, Distance d, x, ... meter m depending on context: “y” for vertical Mass m, M kilogram kg distance, “r” and “R” for radius, etc. Time t second s 2. Notice the meter “m” is plain text while the mass “m” is italicized. Temperature T kelvin K 3. We won’t use candelas or moles. Electric Current I ampere A Luminous Intensity L candela cd Amount of Substance N mole mol SI DERIVED UNITS Quantity Unit Alternate Quantity Symbol Unit Symbol Symbol Area A square meter m2 Volume V cubic meter m3 Speed v meter per second m/s Acceleration a meter per second per second m/s2 m/s/s Force F newton N kg·m/s2 Momentum p kilogram-meter per second kg·m/s N·s Work, Energy, Heat W, E, Q joule J kg·m2/s2 Power P watt W kg·m2/s3 Quantity of Charge Q coulomb C A·s Potential Difference, V volt V J/C Electromotive Force ε Electric Field Strength E newton per coulomb N/C V/m Electric Resistance R ohm ΩV/A Wavelength λ meter m Frequency f, ν hertz Hz 1/s AP Physics Angular Displacement θ radians rad “1” Angular Velocity ω radians per second rad/s Angular Acceleration α radians per second squared rad/s2 Rotational Inertia I kilogram-meter squared kg·m2 Torque τ newton-meter (never “joule”) N·m Angular Momentum L kilogram-meter squared per second kg·m2/s Density D, ρ kilogram per cubic meter kg/m3 Pressure P pascal Pa N/m3 Entropy S joule per kelvin J/K Capacitance C farad F C/V Magnetic Field Strength B tesla T N/A·m Magnetic Flux Φ weber Wb T·m2 The Book of Phyz © Dean Baird. -

CHAPTER 1: Units, Physical Quantities, Dimensions
Lecture 1: Math Preliminaries and Introduction to Vectors 1 CHAPTER 1: Units, Physical Quantities, Dimensions 1. PHYSICS is a science of measurement. The things which are measured are called physical quantities which are defined by the describing how they are to be measured. There are three fundamental quantities in mechanics: Length Mass Time All other physical quantities combinations of these three basic quantities. 2. All physical quantities MUST have units attached to them. The standard system of units is called the SI (Systeme Internationale), or equivalently, the METRIC system. This system uses Length in Meters (m) Mass in Kilograms (kg) Time in Seconds (s) With these abbreviations for the fundamental quantities, one can also be said to be using the MKS system. 3. An example of a derived physical quantity is Density which is the mass per unit volume: Density ≡ Mass = Mass Volume (Length)·(Length)·(Length) 4. Physics uses a lot of formulas and equation. A very powerful tool in working out physics problems with these formulas and equations is Dimensional Analysis. The left side of a formula or equation must have the same dimensions as the right side in terms of the fundamental quantities of mass, length and time. 5. A very important skill to acquire is the art of guesstimation, approximating the answer to a problem. Related to that is an appreciation of sizes. Is the answer to a problem orders of magnitude too big or too small. Lecture 1: Math Preliminaries and Introduction to Vectors 2 The Standards of Length, Mass, and Time The three fundamental physical quantities are length, mass, and time. -

When and Why Are the Values of Physical Quantities Expressed with Uncertainties? Aude Caussarieu, Andrée Tiberghien
When and Why Are the Values of Physical Quantities Expressed with Uncertainties? Aude Caussarieu, Andrée Tiberghien To cite this version: Aude Caussarieu, Andrée Tiberghien. When and Why Are the Values of Physical Quantities Expressed with Uncertainties?: A Case Study of a Physics Undergraduate Laboratory Course. International Journal of Science and Mathematics Education, Springer Verlag, 2016, 15 (6), pp.997-1015. halshs- 01355889 HAL Id: halshs-01355889 https://halshs.archives-ouvertes.fr/halshs-01355889 Submitted on 31 Oct 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 When and Why Are the Values of Physical Quantities Expressed with Uncertainties? A Case Study of a Physics Undergraduate Laboratory Course Aude Caussarieu • Andrée Tiberghien International journal of science and mathematics education, vol Published online 10 march 2016 Abstract The understanding of measurement is related to the understanding of the nature of science—one of the main goals of current international science teaching at all levels of education. This case study explores how a first-year university physics course deals with measurement uncertainties in the light of an epistemological analysis of measurement. The data consist of the course documents, interviews with senior instructors, and laboratory instructors’ responses to an online questionnaire. -
Physics Formulas List
Physics Formulas List Home Physics 95 Physics Formulas 23 Listen Physics Formulas List Learning physics is all about applying concepts to solve problems. This article provides a comprehensive physics formulas list, that will act as a ready reference, when you are solving physics problems. You can even use this list, for a quick revision before an exam. Physics is the most fundamental of all sciences. It is also one of the toughest sciences to master. Learning physics is basically studying the fundamental laws that govern our universe. I would say that there is a lot more to ascertain than just remember and mug up the physics formulas. Try to understand what a formula says and means, and what physical relation it expounds. If you understand the physical concepts underlying those formulas, deriving them or remembering them is easy. This Buzzle article lists some physics formulas that you would need in solving basic physics problems. Physics Formulas Mechanics Friction Moment of Inertia Newtonian Gravity Projectile Motion Simple Pendulum Electricity Thermodynamics Electromagnetism Optics Quantum Physics Derive all these formulas once, before you start using them. Study physics and look at it as an opportunity to appreciate the underlying beauty of nature, expressed through natural laws. Physics help is provided here in the form of ready to use formulas. Physics has a reputation for being difficult and to some extent that's http://www.buzzle.com/articles/physics-formulas-list.html[1/10/2014 10:58:12 AM] Physics Formulas List true, due to the mathematics involved. If you don't wish to think on your own and apply basic physics principles, solving physics problems is always going to be tough. -

Measurements of Electrical Quantities - Ján Šaliga
PHYSICAL METHODS, INSTRUMENTS AND MEASUREMENTS - Measurements Of Electrical Quantities - Ján Šaliga MEASUREMENTS OF ELECTRICAL QUANTITIES Ján Šaliga Department of Electronics and Multimedia Telecommunication, Technical University of Košice, Košice, Slovak Republic Keywords: basic electrical quantities, electric voltage, electric current, electric charge, resistance, capacitance, inductance, impedance, electrical power, digital multimeter, digital oscilloscope, spectrum analyzer, vector signal analyzer, impedance bridge. Contents 1. Introduction 2. Basic electrical quantities 2.1. Electric current and charge 2.2. Electric voltage 2.3. Resistance, capacitance, inductance and impedance 2.4 Electrical power and electrical energy 3. Voltage, current and power representation in time and frequency 3. 1. Time domain 3. 2. Frequency domain 4. Parameters of electrical quantities. 5. Measurement methods and instrumentations 5.1. Voltage, current and resistance measuring instruments 5.1.1. Meters 5.1.2. Oscilloscopes 5.1.3. Spectrum and signal analyzers 5.2. Electrical power and energy measuring instruments 5.2.1. Transition power meters 5.2.2. Absorption power meters 5.3. Capacitance, inductance and resistance measuring instruments 6. Conclusions Glossary Bibliography BiographicalUNESCO Sketch – EOLSS Summary In this work, a SAMPLEfundamental overview of measurement CHAPTERS of electrical quantities is given, including units of their measurement. Electrical quantities are of various properties and have different characteristics. They also differ in the frequency range and spectral content from dc up to tens of GHz and the level range from nano and micro units up to Mega and Giga units. No single instrument meets all these requirements even for only one quantity, and therefore the measurement of electrical quantities requires a wide variety of techniques and instrumentations to perform a required measurement. -

Temperature As a Basic Physical Quantity
Temperature as a basic physical quantity Citation for published version (APA): Boer, de, J. (1965). Temperature as a basic physical quantity. Metrologia, 1(4), 158-169. Document status and date: Published: 01/01/1965 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. If the publication is distributed under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license above, please follow below link for the End User Agreement: www.tue.nl/taverne Take down policy If you believe that this document breaches copyright please contact us at: [email protected] providing details and we will investigate your claim. -

DIMENSIONAL ANALYSIS Ain A
1 The Physical Basis of DIMENSIONAL ANALYSIS Ain A. Sonin Second Edition 2 Copyright © 2001 by Ain A. Sonin Department of Mechanical Engineering MIT Cambridge, MA 02139 First Edition published 1997. Versions of this material have been distributed in 2.25 Advanced Fluid Mechanics and other courses at MIT since 1992. Cover picture by Pat Keck (Untitled, 1992) 3 Contents 1. Introduction 1 2. Physical Quantities and Equations 4 2.1 Physical properties 4 2.2 Physical quantities and base quantities 5 2.3 Unit and numerical value 10 2.4 Derived quantities, dimension, and dimensionless quantities 12 2.5 Physical equations, dimensional homogeneity, and physical constants 15 2.6 Derived quantities of the second kind 19 2.7 Systems of units 22 2.8 Recapitulation 27 3. Dimensional Analysis 29 3.1 The steps of dimensional analysis and Buckingham’s Pi-Theorem 29 Step 1: The independent variables 29 Step 2: Dimensional considerations 30 Step 3: Dimensional variables 32 Step 4: The end game and Buckingham’s P -theorem 32 3.2 Example: Deformation of an elastic sphere striking a wall 33 Step 1: The independent variables 33 Step 2: Dimensional considerations 35 Step 3: Dimensionless similarity parameters 36 Step 4: The end game 37 3.2 On the utility of dimensional analysis and some difficulties and questions that arise in its application 37 Similarity 37 Out-of-scale modeling 38 Dimensional analysis reduces the number of variables and minimizes work. 38 4 An incomplete set of independent quantities may destroy the analysis 40 Superfluous independent quantities complicate the result unnecessarily 40 On the importance of simplifying assumptions 41 On choosing a complete set of independent variables 42 The result is independent of how one chooses a dimensionally independent subset 43 The result is independent of the type of system of units 43 4. -

DO WE KNOW WHAT the TEMPERATURE IS? Jiří J
DO WE KNOW WHAT THE TEMPERATURE IS? Jiří J. Mareš1 Institute of Physics of the ASCR, v. v. i., Cukrovarnická 10, 162 00, Prague 6, Czech Republic Abstract: Temperature, the central concept of thermal physics, is one of the most frequently employed physical quantities in common practice. Even though the operative methods of the temperature measurement are described in detail in various practical instructions and textbooks, the rigorous treatment of this concept is almost lacking in the current literature. As a result, the answer to a simple question of “what the temperature is” is by no means trivial and unambiguous. There is especially an appreciable gap between the temperature as introduced in the frame of statistical theory and the only experimentally observable quantity related to this concept, phenomenological temperature. Just the logical and epistemological analysis of the present concept of phenomenological temperature is the kernel of the contribution. Keywords: thermodynamics, temperature scales, hotness, Mach’s postulates, Carnot’s principle, Kelvin’s proposition Introduction What does the temperature mean? It is a classically simple question astonishingly lacking an appropriate answer. The answers, namely, which can be found in the textbooks on thermodynamics, are often hardly acceptable without serious objections. For illustration, let us give a few typical examples here, the reader can easily find others in the current literature by himself. To the most hand-waving belong the statements such as “the temperature is known from the basic courses of physics” or even “temperature is known intuitively”. More frankly sounds the widely used operative definition “the temperature is reading on the scale of thermometer”.