CHAPTER 1: Units, Physical Quantities, Dimensions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How Long Is a Year.Pdf
How Long Is A Year? Dr. Bryan Mendez Space Sciences Laboratory UC Berkeley Keeping Time The basic unit of time is a Day. Different starting points: • Sunrise, • Noon, • Sunset, • Midnight tied to the Sun’s motion. Universal Time uses midnight as the starting point of a day. Length: sunrise to sunrise, sunset to sunset? Day Noon to noon – The seasonal motion of the Sun changes its rise and set times, so sunrise to sunrise would be a variable measure. Noon to noon is far more constant. Noon: time of the Sun’s transit of the meridian Stellarium View and measure a day Day Aday is caused by Earth’s motion: spinning on an axis and orbiting around the Sun. Earth’s spin is very regular (daily variations on the order of a few milliseconds, due to internal rearrangement of Earth’s mass and external gravitational forces primarily from the Moon and Sun). Synodic Day Noon to noon = synodic or solar day (point 1 to 3). This is not the time for one complete spin of Earth (1 to 2). Because Earth also orbits at the same time as it is spinning, it takes a little extra time for the Sun to come back to noon after one complete spin. Because the orbit is elliptical, when Earth is closest to the Sun it is moving faster, and it takes longer to bring the Sun back around to noon. When Earth is farther it moves slower and it takes less time to rotate the Sun back to noon. Mean Solar Day is an average of the amount time it takes to go from noon to noon throughout an orbit = 24 Hours Real solar day varies by up to 30 seconds depending on the time of year. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Physics, Chapter 1: Fundamental Quantities
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Robert Katz Publications Research Papers in Physics and Astronomy 1-1958 Physics, Chapter 1: Fundamental Quantities Henry Semat City College of New York Robert Katz University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/physicskatz Part of the Physics Commons Semat, Henry and Katz, Robert, "Physics, Chapter 1: Fundamental Quantities" (1958). Robert Katz Publications. 137. https://digitalcommons.unl.edu/physicskatz/137 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Robert Katz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Part One MECHANICS 1 Fundamental Quantities 1-1 The Scope of Physics Physics is a fundamental science dealing with matter and energy. By convention, the subject matter of physics has been divided into such topics as mechanics, heat, sound, light, and electricity. In addition to these general classifications, present-day physics includes atomic physics, nu clear physics, solid-state physics, chemical physics, biophysics, and many other subdivisions. It is impossible to include all aspects of physics in a single definition or paragraph, and to distinguish physics clearly from its nearest neighbors, the other physical sciences-astronomy., chemistry, and geology. Like other scientists, the physicist studies nature, and, although the scientist is himself part of the world, he attempts to describe nature as it exists without his interference. Inspired by the conviction that nature is orderly and "rational," the physicist has sought to find that order and to express it as elegantly and as concisely as possible. -

International System of Units (SI)
International System of Units (SI) National Institute of Standards and Technology (www.nist.gov) Multiplication Prefix Symbol factor 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 * hecto h 101 * deka da 10-1 * deci d 10-2 * centi c 10-3 milli m 10-6 micro µ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a * Prefixes of 100, 10, 0.1, and 0.01 are not formal SI units From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Seven basic SI units Physical quantity Unit Symbol Length meter m Mass kilogram kg Time second s Electrical current ampere A Thermodynamic temperature kelvin K Amount of substance mole mol Luminous intensity candela cd Supplementary SI units Physical quantity Unit Symbol Plane angle radian rad Solid angle steradian sr From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Derived SI units with special names Physical quantity Unit Symbol Derivation Absorbed dose gray Gy J kg-1 Capacitance farad F A s V-1 Conductance siemens S A V-1 Disintegration rate becquerel Bq l s-1 Electrical charge coulomb C A s Electrical potential volt V W A-1 Energy joule J N m Force newton N kg m s-2 Illumination lux lx lm m-2 Inductance henry H V s A-1 Luminous flux lumen lm cd sr Magnetic flux weber Wb V s Magnetic flux density tesla T Wb m-2 Pressure pascal Pa N m-2 Power watt W J s-1 Resistance ohm Ω V A-1 Volume liter L dm3 From: Downs, R.J. -

Terminology of Geological Time: Establishment of a Community Standard
Terminology of geological time: Establishment of a community standard Marie-Pierre Aubry1, John A. Van Couvering2, Nicholas Christie-Blick3, Ed Landing4, Brian R. Pratt5, Donald E. Owen6 and Ismael Ferrusquía-Villafranca7 1Department of Earth and Planetary Sciences, Rutgers University, Piscataway NJ 08854, USA; email: [email protected] 2Micropaleontology Press, New York, NY 10001, USA email: [email protected] 3Department of Earth and Environmental Sciences and Lamont-Doherty Earth Observatory of Columbia University, Palisades NY 10964, USA email: [email protected] 4New York State Museum, Madison Avenue, Albany NY 12230, USA email: [email protected] 5Department of Geological Sciences, University of Saskatchewan, Saskatoon SK7N 5E2, Canada; email: [email protected] 6Department of Earth and Space Sciences, Lamar University, Beaumont TX 77710 USA email: [email protected] 7Universidad Nacional Autónomo de México, Instituto de Geologia, México DF email: [email protected] ABSTRACT: It has been recommended that geological time be described in a single set of terms and according to metric or SI (“Système International d’Unités”) standards, to ensure “worldwide unification of measurement”. While any effort to improve communication in sci- entific research and writing is to be encouraged, we are also concerned that fundamental differences between date and duration, in the way that our profession expresses geological time, would be lost in such an oversimplified terminology. In addition, no precise value for ‘year’ in the SI base unit of second has been accepted by the international bodies. Under any circumstances, however, it remains the fact that geologi- cal dates – as points in time – are not relevant to the SI. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -
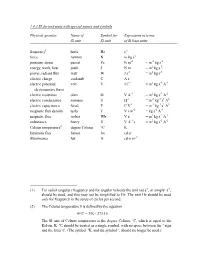
1.4.3 SI Derived Units with Special Names and Symbols
1.4.3 SI derived units with special names and symbols Physical quantity Name of Symbol for Expression in terms SI unit SI unit of SI base units frequency1 hertz Hz s-1 force newton N m kg s-2 pressure, stress pascal Pa N m-2 = m-1 kg s-2 energy, work, heat joule J N m = m2 kg s-2 power, radiant flux watt W J s-1 = m2 kg s-3 electric charge coulomb C A s electric potential, volt V J C-1 = m2 kg s-3 A-1 electromotive force electric resistance ohm Ω V A-1 = m2 kg s-3 A-2 electric conductance siemens S Ω-1 = m-2 kg-1 s3 A2 electric capacitance farad F C V-1 = m-2 kg-1 s4 A2 magnetic flux density tesla T V s m-2 = kg s-2 A-1 magnetic flux weber Wb V s = m2 kg s-2 A-1 inductance henry H V A-1 s = m2 kg s-2 A-2 Celsius temperature2 degree Celsius °C K luminous flux lumen lm cd sr illuminance lux lx cd sr m-2 (1) For radial (angular) frequency and for angular velocity the unit rad s-1, or simply s-1, should be used, and this may not be simplified to Hz. The unit Hz should be used only for frequency in the sense of cycles per second. (2) The Celsius temperature θ is defined by the equation θ/°C = T/K - 273.15 The SI unit of Celsius temperature is the degree Celsius, °C, which is equal to the Kelvin, K. -

Calendar As a Criterion in the Study of Culture
Article Calendar as a Criterion in the Study of Culture Marija Šegan-Radonji´c 1,‡ and Stevo Šegan 2,‡ 1 Mathematical Institute of the Serbian Academy of Sciences and Arts 2 Department of Mathematics, State University of Novi Pazar Abstract: The paper considers the calendar as a link between the cosmos and mankind, and it introduces it as an instrument in studying culture. It uses the concept of calendars/calendar systems as a criterion for recognition and formation of culture in general. Starting from an assumption that the calendar is a structurally organized system of events or holidays, it analyses the basic units of a calendar: day, month and year, and distinguishes a calendar holiday from a non-calendar holiday. It states that the calendars are a structural list of collective memory within a social group, where this memory is described in cyclical categories – calendar holidays. Furthermore, considering that the initial epoch of year counting may be different in different cultures, it discusses how cultural self-awareness is expressed through the epoch of the calendar era. Finally, it explores how and to what extent the formation, interaction, and reforms of calendars and their systems reflect the change in culture. The paper concludes that calendars and their systems should be used as a criterion in defining culture. Keywords: Calendar; Culture. 1. Introduction Culture, as a notion, can be considered in a narrower and a broader sense1, and some theoreticians have succeeded to identify as many as 164 definitions of this phenomenon [2]. Furthermore, modern science strives to comprehend and describe this phenomenon as generally as possible, and the new complex disciplines, such as the problem of culture typology (criterion) [3], have appeared and been developed. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

The Wilkie Way Newsletter July/August 2020 New Website
The Wilkie Way Newsletter July/August 2020 www.wilkieway.co.nz New Website The new website went live last Friday. There is a slight change in the address having dropped the word “the”from before the words wilkieway. www.wilkieway.co.nz However if you use the old address you will still be able to follow a link to the the new site. The home page is clean and simple with links to Education consultancy, the online store, information on Maths Aotearoa/Pearson Maths (with a link through to Edify website who sell these books. I am no longer selling these books direct). Workplace training & assessment is for NZQA adult education and training - my husbands arm of the business. Maths, News and information links to a blog which will shortly be up and running. The Membership Area is for those who have paid a subscription. The subscription can be bought from the online store - an individual subscription is $45.00 Once you have bought the subscription, go to the members area and create an account. This account then needs to be activated by me. I will endeavour to do this as soon as possible after I receive the email telling me of a new sign up. (Normally within 12 hours and if I am at the computer it could be immediately - I will send an email to let you know it is activated) The transfer of current membership did not go as smoothly as promised. All current school memberships have been set up manually and the school contact has been sent an email with new login details. -

EL Light Output Definition.Pdf
GWENT GROUP ADVANCED MATERIAL SYSTEMS Luminance The candela per square metre (cd/m²) is the SI unit of luminance; nit is a non-SI name also used for this unit. It is often used to quote the brightness of computer displays, which typically have luminance’s of 50 to 300 nits (the sRGB spec for monitor’s targets 80 nits). Modern flat-panel (LCD and plasma) displays often exceed 300 cd/m² or 300 nits. The term is believed to come from the Latin "nitere" = to shine. Candela per square metre 1 Kilocandela per square metre 10-3 Candela per square centimetre 10-4 Candela per square foot 0,09 Foot-lambert 0,29 Lambert 3,14×10-4 Nit 1 Stilb 10-4 Luminance is a photometric measure of the density of luminous intensity in a given direction. It describes the amount of light that passes through or is emitted from a particular area, and falls within a given solid angle. The SI unit for luminance is candela per square metre (cd/m2). The CGS unit of luminance is the stilb, which is equal to one candela per square centimetre or 10 kcd/m2 Illuminance The lux (symbol: lx) is the SI unit of illuminance and luminous emittance. It is used in photometry as a measure of the intensity of light, with wavelengths weighted according to the luminosity function, a standardized model of human brightness perception. In English, "lux" is used in both singular and plural. Microlux 1000000 Millilux 1000 Lux 1 Kilolux 10-3 Lumen per square metre 1 Lumen per square centimetre 10-4 Foot-candle 0,09 Phot 10-4 Nox 1000 In photometry, illuminance is the total luminous flux incident on a surface, per unit area. -

Can I Change an Activity from an 8-Hour Workday to a 10-Hour Workday in My Schedule? During a Primavera P6 Training Class This M
Can I change an activity from an 8-hour workday to a 10-hour workday in my schedule? During a Primavera P6 training class this month, a student asked me: “If a client wants to see the difference in the construction schedule between an 8-hour work week and a ten-hour work week how can we show that if our durations are in days?” We had a long discussion on how schedules are used and created by all the stakeholders and how a project manager or superintendent should set up the schedule to be most useful as the project progresses. As a background note about Primavera P6, most planner and schedulers will set their default durations to days but the software calculates all durations in hours and minutes. During the development of a project schedule the subcontractors will typically plan their scopes of work in days and/or weeks as they work through a building. As they, subcontractors, submit their schedule either in Microsoft Project or Primavera P6 the General Contractor (GC) will incorporate or create a schedule based on those durations and relationships. Typically, we see subcontractors submit schedules with activity durations identified as days and their calendars are typically a five-day with eight hours per day for working. Hopefully the durations for each activity are less than five-day increments if we plan to update our schedule on a weekly basis. The GC will take that list of activities and merge them into the master schedule along with the remaining subcontractors’ schedules to create a new baseline schedule.