Sonic Hedgehog Regulates Integrin Activity, Cadherin Contacts, and Cell Polarity to Orchestrate Neural Tube Morphogenesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanical Forces Induce an Asthma Gene Signature in Healthy Airway Epithelial Cells Ayşe Kılıç1,10, Asher Ameli1,2,10, Jin-Ah Park3,10, Alvin T
www.nature.com/scientificreports OPEN Mechanical forces induce an asthma gene signature in healthy airway epithelial cells Ayşe Kılıç1,10, Asher Ameli1,2,10, Jin-Ah Park3,10, Alvin T. Kho4, Kelan Tantisira1, Marc Santolini 1,5, Feixiong Cheng6,7,8, Jennifer A. Mitchel3, Maureen McGill3, Michael J. O’Sullivan3, Margherita De Marzio1,3, Amitabh Sharma1, Scott H. Randell9, Jefrey M. Drazen3, Jefrey J. Fredberg3 & Scott T. Weiss1,3* Bronchospasm compresses the bronchial epithelium, and this compressive stress has been implicated in asthma pathogenesis. However, the molecular mechanisms by which this compressive stress alters pathways relevant to disease are not well understood. Using air-liquid interface cultures of primary human bronchial epithelial cells derived from non-asthmatic donors and asthmatic donors, we applied a compressive stress and then used a network approach to map resulting changes in the molecular interactome. In cells from non-asthmatic donors, compression by itself was sufcient to induce infammatory, late repair, and fbrotic pathways. Remarkably, this molecular profle of non-asthmatic cells after compression recapitulated the profle of asthmatic cells before compression. Together, these results show that even in the absence of any infammatory stimulus, mechanical compression alone is sufcient to induce an asthma-like molecular signature. Bronchial epithelial cells (BECs) form a physical barrier that protects pulmonary airways from inhaled irritants and invading pathogens1,2. Moreover, environmental stimuli such as allergens, pollutants and viruses can induce constriction of the airways3 and thereby expose the bronchial epithelium to compressive mechanical stress. In BECs, this compressive stress induces structural, biophysical, as well as molecular changes4,5, that interact with nearby mesenchyme6 to cause epithelial layer unjamming1, shedding of soluble factors, production of matrix proteins, and activation matrix modifying enzymes, which then act to coordinate infammatory and remodeling processes4,7–10. -

Role of Sonic Hedgehog Signaling Pathway in Neuroblastoma Development
Review Article Biology and Medicine, 1 (4): Rev2, 2009 eISSN: 09748369, www.biolmedonline.com Role of Sonic hedgehog signaling pathway in neuroblastoma development Mehdi Hayat Shahi1,2,3,§, Subrata Sinha2, *Mohammad Afzal3, *Javier S Castresana1 1Unidad de Biologia de Tumoures Cerebrales, Universidad de Navarra, 31008 Pamplona, Spain. 2Department of Biochemistry, All India Institute of Medical Sciences (AIIMS), New Delhi-110029, India. 3Section of Genetics, Department of Zoology, Aligarh Muslim University, Aligarh-202002, India. §Present address: Department of Genetics and Pathology, Rudbeck Laboratory, University of Uppsala, Uppsala- 75185, Sweden. *Corresponding Authors: Javier S Castresana, [email protected] Mohammad Afzal, [email protected] Abstract Malignant transformation of normal cells is a complex and accumulative process. Understanding this event gives insight into mechanisms of developmental biology and physical interaction of cellular machinery with surrounding ambient factors. However, the trend of embryonic malignancy is not interactive with ambient factors, rather a cause of deregulations of internal developmental process. In this review, we have attempted to explore the possibility of Sonic hedgehog role (Shh) in the development of neuroblastoma tumour. It is the major extra cranial tumour and develops in very early stage of childhood. Sonic hedgehog signaling is very well studied in another major childhood tumour i.e. medulloblastoma that contributes 20-25% of childhood tumours, and one-fourth of medulloblastoma is due to abnormality in the Shh signaling pathway. Therefore, we would consider whether Shh could also contribute to the development of neuroblastoma. Although scientists are coming up with the role of Shh in the neuroblastoma, the Sonic hedgehog signaling is very much one of the promising pathways because of its multi-dimensional role not only in CNS development but also in organogenesis and other major tumour development. -

Sonic Hedgehog Signaling Limits Atopic Dermatitis Via Gli2-Driven Immune Regulation
Sonic Hedgehog signaling limits atopic dermatitis via Gli2-driven immune regulation Eleftheria Papaioannou, … , Ryan F. L. O’Shaughnessy, Tessa Crompton J Clin Invest. 2019. https://doi.org/10.1172/JCI125170. Research Article Immunology Inflammation Hedgehog (Hh) proteins regulate development and tissue homeostasis, but their role in atopic dermatitis (AD) remains unknown. We found that on induction of mouse AD, Sonic Hedgehog (Shh) expression in skin, and Hh pathway action in skin T cells were increased. Shh signaling reduced AD pathology and the levels of Shh expression determined disease severity. Hh-mediated transcription in skin T cells in AD-induced mice increased Treg populations and their suppressive function through increased active transforming growth factor–b (TGF-b) in Tregs signaling to skin T effector populations to reduce disease progression and pathology. RNA sequencing of skin CD4+ T cells from AD-induced mice demonstrated that Hh signaling increased expression of immunoregulatory genes and reduced expression of inflammatory and chemokine genes. Addition of recombinant Shh to cultures of naive human CD4+ T cells in iTreg culture conditions increased FOXP3 expression. Our findings establish an important role for Shh upregulation in preventing AD, by increased Gli-driven Treg cell–mediated immune suppression, paving the way for a potential new therapeutic strategy. Find the latest version: http://jci.me/125170/pdf The Journal of Clinical Investigation RESEARCH ARTICLE Sonic Hedgehog signaling limits atopic dermatitis via Gli2-driven immune regulation Eleftheria Papaioannou,1 Diana C. Yánez,1,2 Susan Ross,1 Ching-In Lau,1 Anisha Solanki,1 Mira Manilal Chawda,1 Alex Virasami,1 Ismael Ranz,3 Masahiro Ono,1,4 Ryan F. -

1714 Gene Comprehensive Cancer Panel Enriched for Clinically Actionable Genes with Additional Biologically Relevant Genes 400-500X Average Coverage on Tumor
xO GENE PANEL 1714 gene comprehensive cancer panel enriched for clinically actionable genes with additional biologically relevant genes 400-500x average coverage on tumor Genes A-C Genes D-F Genes G-I Genes J-L AATK ATAD2B BTG1 CDH7 CREM DACH1 EPHA1 FES G6PC3 HGF IL18RAP JADE1 LMO1 ABCA1 ATF1 BTG2 CDK1 CRHR1 DACH2 EPHA2 FEV G6PD HIF1A IL1R1 JAK1 LMO2 ABCB1 ATM BTG3 CDK10 CRK DAXX EPHA3 FGF1 GAB1 HIF1AN IL1R2 JAK2 LMO7 ABCB11 ATR BTK CDK11A CRKL DBH EPHA4 FGF10 GAB2 HIST1H1E IL1RAP JAK3 LMTK2 ABCB4 ATRX BTRC CDK11B CRLF2 DCC EPHA5 FGF11 GABPA HIST1H3B IL20RA JARID2 LMTK3 ABCC1 AURKA BUB1 CDK12 CRTC1 DCUN1D1 EPHA6 FGF12 GALNT12 HIST1H4E IL20RB JAZF1 LPHN2 ABCC2 AURKB BUB1B CDK13 CRTC2 DCUN1D2 EPHA7 FGF13 GATA1 HLA-A IL21R JMJD1C LPHN3 ABCG1 AURKC BUB3 CDK14 CRTC3 DDB2 EPHA8 FGF14 GATA2 HLA-B IL22RA1 JMJD4 LPP ABCG2 AXIN1 C11orf30 CDK15 CSF1 DDIT3 EPHB1 FGF16 GATA3 HLF IL22RA2 JMJD6 LRP1B ABI1 AXIN2 CACNA1C CDK16 CSF1R DDR1 EPHB2 FGF17 GATA5 HLTF IL23R JMJD7 LRP5 ABL1 AXL CACNA1S CDK17 CSF2RA DDR2 EPHB3 FGF18 GATA6 HMGA1 IL2RA JMJD8 LRP6 ABL2 B2M CACNB2 CDK18 CSF2RB DDX3X EPHB4 FGF19 GDNF HMGA2 IL2RB JUN LRRK2 ACE BABAM1 CADM2 CDK19 CSF3R DDX5 EPHB6 FGF2 GFI1 HMGCR IL2RG JUNB LSM1 ACSL6 BACH1 CALR CDK2 CSK DDX6 EPOR FGF20 GFI1B HNF1A IL3 JUND LTK ACTA2 BACH2 CAMTA1 CDK20 CSNK1D DEK ERBB2 FGF21 GFRA4 HNF1B IL3RA JUP LYL1 ACTC1 BAG4 CAPRIN2 CDK3 CSNK1E DHFR ERBB3 FGF22 GGCX HNRNPA3 IL4R KAT2A LYN ACVR1 BAI3 CARD10 CDK4 CTCF DHH ERBB4 FGF23 GHR HOXA10 IL5RA KAT2B LZTR1 ACVR1B BAP1 CARD11 CDK5 CTCFL DIAPH1 ERCC1 FGF3 GID4 HOXA11 IL6R KAT5 ACVR2A -
Type of the Paper (Article
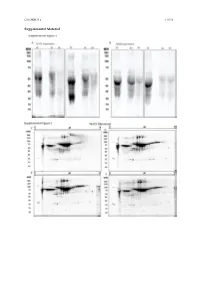
Cells 2020, 9, x 1 of 19 Supplemental Material Cells 2020, 9, x 2 of 19 Figure S1. Secretome enrichment: protocol optimization. 1D SDS-PAGE documentation of washing steps: Culture medium was substituted with FCS-free medium, which was changed every 2 h. The supernatants were then collected, and the proteins isolated and separated in 1D SDS-PAGE ((A) TK173 and (B) TK188). Proteins were stained with Flamingo fluorescent gel stain. Two-dimensional pattern of the proteins isolated from supernatant of TK173, (C) 2 h, (D) 4 h, (E) 6 h, and (F) 8 h after changing to FCS-free medium. (G) Cell secretome collected 24 h after elimination of the contaminating FCS-proteins with different washing steps. Proteins were stained with Flamingo fluorescent gel stain. Cells 2020, 9, x 3 of 19 Figure S2. 2-DE reference maps of secretomes; 150 μg proteins were loaded on an 11 cm IPG strip with a linear pH gradient PI 5–8 for IEF; 12% SDS-polyacrylamide gels were used for the second dimension. Proteins were stained with Flamingo fluorescent gel stain. Identified spots were assigned a number corresponding to that in their table. 2-DE maps from secretome of (A) TK173 control and (B) TGFβ1- treated ones. The 2-DE patterns revealed an alteration of secretome in stimulated TK173. Secretome patterns from TK173 treated with (C) ANG II and (D) PDGF. Cells 2020, 9, x 4 of 19 A Figure S3. Classification of the differentially expressed proteins upon ANG II, TGFβ1, or PDGF treatment in TK173. (A) Bar charts of the cellular component analyzed by STRAP biological function analysis in which the identified proteins from all treatments in both cell types are involved. -

Figure S1. HAEC ROS Production and ML090 NOX5-Inhibition
Figure S1. HAEC ROS production and ML090 NOX5-inhibition. (a) Extracellular H2O2 production in HAEC treated with ML090 at different concentrations and 24 h after being infected with GFP and NOX5-β adenoviruses (MOI 100). **p< 0.01, and ****p< 0.0001 vs control NOX5-β-infected cells (ML090, 0 nM). Results expressed as mean ± SEM. Fold increase vs GFP-infected cells with 0 nM of ML090. n= 6. (b) NOX5-β overexpression and DHE oxidation in HAEC. Representative images from three experiments are shown. Intracellular superoxide anion production of HAEC 24 h after infection with GFP and NOX5-β adenoviruses at different MOIs treated or not with ML090 (10 nM). MOI: Multiplicity of infection. Figure S2. Ontology analysis of HAEC infected with NOX5-β. Ontology analysis shows that the response to unfolded protein is the most relevant. Figure S3. UPR mRNA expression in heart of infarcted transgenic mice. n= 12-13. Results expressed as mean ± SEM. Table S1: Altered gene expression due to NOX5-β expression at 12 h (bold, highlighted in yellow). N12hvsG12h N18hvsG18h N24hvsG24h GeneName GeneDescription TranscriptID logFC p-value logFC p-value logFC p-value family with sequence similarity NM_052966 1.45 1.20E-17 2.44 3.27E-19 2.96 6.24E-21 FAM129A 129. member A DnaJ (Hsp40) homolog. NM_001130182 2.19 9.83E-20 2.94 2.90E-19 3.01 1.68E-19 DNAJA4 subfamily A. member 4 phorbol-12-myristate-13-acetate- NM_021127 0.93 1.84E-12 2.41 1.32E-17 2.69 1.43E-18 PMAIP1 induced protein 1 E2F7 E2F transcription factor 7 NM_203394 0.71 8.35E-11 2.20 2.21E-17 2.48 1.84E-18 DnaJ (Hsp40) homolog. -

Origin, Characterization and Roles of Matrix Vesicles in Physiological and Pathological Mineralization
Origin, characterization and roles of matrix vesicles in physiological and pathological mineralization. Cyril Thouverey To cite this version: Cyril Thouverey. Origin, characterization and roles of matrix vesicles in physiological and pathological mineralization.. Life Sciences [q-bio]. Université Claude Bernard - Lyon I, 2008. English. tel- 00304214 HAL Id: tel-00304214 https://tel.archives-ouvertes.fr/tel-00304214 Submitted on 22 Jul 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N° d’ordre: 95-2008 Année 2008 THESE Présentée devant Nencki Institute of Experimental Biology, Polish Academy of Sciences Université Claude Bernard Lyon 1, Pour l’obtention Du DIPLOME DE DOCTORAT (Arrêté du 7 août 2006 et arrêté du 6 janvier 2005) Présentée et soutenue publiquement le 20 juin 2008 Par Cyril Thouverey Origin, characterization and roles of matrix vesicles in physiological and pathological mineralization Directeurs de thèse: Pr René Buchet et Pr Sławomir Pikuła JURY: Mme Françoise Bleicher Mme Joanna Bandorowicz-Pikuła Mme Martime Cohen-Solal Mr René Buchet Mr Sławomir Pikuła Mr Aleksander Sikorski UNIVERSITE CLAUDE BERNARD - LYON I Président de l’Université M. le Professeur L. COLLET Vice-Président du Conseil Scientifique M. -

Expression of Primary Cilia-Related Genes in Developing Mouse Gonads RAFAL P
Int. J. Dev. Biol. 63: 615-621 (2019) https://doi.org/10.1387/ijdb.190049rp www.intjdevbiol.com Expression of primary cilia-related genes in developing mouse gonads RAFAL P. PIPREK*,1, DAGMARA PODKOWA1, MALGORZATA KLOC2,3,4 and JACEK Z. KUBIAK5,6 1Department of Comparative Anatomy, Institute of Zoology and Biomedical Research, Jagiellonian University, Krakow, Poland, 2The Houston Methodist Research Institute, Houston, TX, USA, 3Department of Surgery, The Houston Methodist Hospital, Houston TX, USA, 4University of Texas, MD Anderson Cancer Center, Houston TX, USA, 5Univ Rennes, CNRS, Institute of Genetics and Development of Rennes, UMR 6290, Cell Cycle Group, Faculty of Medicine, Rennes, France and 6Laboratory of Regenerative Medicine and Cell Biology, Military Institute of Hygiene and Epidemiology (WIHE), Warsaw, Poland ABSTRACT Mechanisms governing differentiation of the bipotential gonad into the testes or ovaries are complex and still vague. The primary cilium is an organelle involved in cell signaling, which controls the development of many organs, but the role of primary cilium in the sex determination and sexual differentiation of gonads is completely unknown. Here we studied the expression of genes involved in primary cilium formation and functioning in fetal mouse gonads, before, during and after sexual differentiation. We studied the expression of 175 primary cilia-related genes using microarray technique. 144 of these genes were ubiquitously expressed in all studied cell types with no significant differences in expression level. Such a high level of expression of primary cilia-related genes in developing mouse gonads suggests that the primary cilia and/or primary cilia-related genes are important for the development of both somatic and germline component of the gonads. -

Nuclear Factor-KB Contributes to Hedgehog Signaling Pathway Activation Through Sonic Hedgehog Induction in Pancreatic Cancer
Research Article Nuclear Factor-KB Contributes to Hedgehog Signaling Pathway Activation through Sonic Hedgehog Induction in Pancreatic Cancer Hiroshi Nakashima,1 Masafumi Nakamura,1 Hiroshi Yamaguchi,2 Naoki Yamanaka,1 Takashi Akiyoshi,1 Kenichiro Koga,1 Koji Yamaguchi,3 Masazumi Tsuneyoshi,2 Masao Tanaka,3 and Mitsuo Katano1 Departments of 1Cancer Therapy and Research, 2Anatomic Pathology, and 3Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan Abstract The hedgehog (Hh) signaling pathway is crucial to growth and The hedgehog (Hh) signaling pathway, which functions as an patterning in a wide variety of tissues during embryonic organizer in embryonic development, is implicated in the development (3, 4). Of three Hh ligands, sonic Hh (Shh), Indian development of various tumors. In pancreatic cancer, pathway Hh (Ihh), and desert Hh (Dhh), Shh is reported to play an essential activation is reported to result from aberrant expression of role in the development of pancreatic cancer as well as pancreatic organogenesis (5, 6). Shh undergoes extensive post-translational the ligand, sonic Hh (Shh). However, the details of the mechanisms regulating Shh expression are not yet known. modifications to become biologically active (7). The signal peptide We hypothesized that nuclear factor-KB (NF-KB), a hallmark is cleaved from the precursor form of Shh to yield the 45-kDa transcription factor in inflammatory responses, contributes to full-length form. Further processing of this molecular form of Shh the overexpression of Shh in pancreatic cancer. In the present generates a 19-kDa amino and 26-kDa COOH-terminal peptides. study, we found a close positive correlation between NF-KB The 19-kDa NH2-terminal peptide undergoes COOH-terminal p65 and Shh expression in surgically resected pancreas esterification to a cholesterol molecule before being secreted into specimens, including specimens of chronic pancreatitis and the extracellular spaces where it mediates the physiologic actions pancreatic adenocarcinoma. -

The Sonic Hedgehog Signaling System As a Bistable Genetic Switch
2748 Biophysical Journal Volume 86 May 2004 2748–2757 The Sonic Hedgehog Signaling System as a Bistable Genetic Switch Karen Lai, Matthew J. Robertson, and David V. Schaffer Department of Chemical Engineering and the Helen Wills Neuroscience Institute, University of California, Berkeley, California ABSTRACT Sonic hedgehog (Shh) controls critical cellular decisions between distinct fates in many systems, particularly in stem cells. The Shh network functions as a genetic switch, and we have theoretically and computationally analyzed how its structure can endow it with the ability to switch fate choices at a threshold Shh concentration. The network is composed of a positive transcriptional feedback loop embedded within a negative signaling feedback loop. Specifically, positive feedback by the transcription factor Gli, which upregulates its own expression, leads to a switch that can adopt two distinct states as a function of Shh. However, Gli also upregulates the signaling repressor Patched, negative feedback that reins in the strong Gli autoregulatory loop. Mutations that have been associated with cancer are predicted to yield an irreversible switch to a high Gli state. Finally, stochastic simulation reveals the negative Patched feedback loop serves a critical function of dampening Gli fluctuations to reduce spontaneous state switching and preserve the network’s robust, switch-like behavior. Tightly linked positive and negative feedback loops are present in many signaling systems, and the Shh system is therefore likely representative of a large set of gene regulation networks that control stem cell fate throughout development and into adulthood. INTRODUCTION Throughout development and adulthood, cells are exposed to development by forming a concentration gradient, a phe- complex regulatory signals and must correctly interpret these nomenon best characterized in the neural tube and spinal signals to implement necessary functional decisions. -

Functional Study of Mir-27A in Human Hepatic Stellate Cells by Proteomic Analysis: Comprehensive View and a Role in Myogenic Tans-Differentiation
Functional Study of miR-27a in Human Hepatic Stellate Cells by Proteomic Analysis: Comprehensive View and a Role in Myogenic Tans-Differentiation Yuhua Ji1, Jinsheng Zhang2, Wenwen Wang3, Juling Ji3* 1 Key Laboratory of Neuroregeneration, Nantong University, Nanton, China, 2 Department of Pathology, Shanghai Medical College, Fudan University, Shanghai, PR China, 3 Department of Pathology, Medical School of Nantong University, Nantong, PR China Abstract We previous reported that miR-27a regulates lipid metabolism and cell proliferation during hepatic stellate cells (HSCs) activation. To further explore the biological function and underlying mechanisms of miR-27a in HSCs, global protein expression affected by overexpression of miR-27a in HSCs was analyzed by a cleavable isotope-coded affinity tags (cICAT) based comparative proteomic approach. In the present study, 1267 non-redundant proteins were identified with unique accession numbers (score $1.3, i.e. confidence $95%), among which 1171 were quantified and 149 proteins (12.72%) were differentially expressed with a differential expression ratio of 1.5. We found that up-regulated proteins by miR-27a mainly participate in cell proliferation and myogenesis, while down-regulated proteins were the key enzymes involved in de novo lipid synthesis. The expression of a group of six miR-27a regulated proteins was validated and the function of one miR-27a regulated protein was further validated. The results not only delineated the underlying mechanism of miR-27a in modulating fat metabolism and cell proliferation, but also revealed a novel role of miR-27a in promoting myogenic tans- differentiation during HSCs activation. This study also exemplified proteomics strategy as a powerful tool for the functional study of miRNA. -

The Hedgehog Signaling Pathway Promotes Chemotherapy Resistance Via Multidrug Resistance Protein 1 in Ovarian Cancer
2610 ONCOLOGY REPORTS 44: 2610-2620, 2020 The Hedgehog signaling pathway promotes chemotherapy resistance via multidrug resistance protein 1 in ovarian cancer HONG ZHANG1*, LANYAN HU1*, MINZHANG CHENG2, QIAN WANG1, XINYUE HU1 and QI CHEN1 1Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Nanchang University; 2Center for Experimental Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, P.R. China Received February 7, 2020; Accepted July 14, 2020 DOI: 10.3892/or.2020.7798 Abstract. Various studies have revealed that the Hedgehog (Hh) regulated by the Hh signaling pathway and is likely a down- signaling pathway promotes ovarian cancer invasion, migra- stream transcription factor of Gli2. In conclusion, targeting tion and drug resistance. Previous studies by our group have the Hh signaling pathway increases the sensitivity of ovarian identified a set of genes, including multidrug resistance gene 1 cancer to DDP. MDR1 is a target gene of the Hh signaling (MDR1), that are regulated by Hh signaling in ovarian cancer. pathway and this pathway may affect chemoresistance of However, the association between Hh signaling activation and ovarian cancer to DDP via MDR1. MDR1 expression requires further validation. In the present study, reverse transcription-quantitative PCR or western blot Introduction assays were used to evaluate the mRNA and protein expression levels of MDR1, Sonic Hh (Shh), glioma-associated oncogene Ovarian cancer is the most fatal type of tumor of the female 2 (Gli2), Gli1 and γ-phosphorylated H2A.X variant histone genital tract (1). Although surgical techniques and chemo- (γ‑H2AX). MTT and colony‑formation assays were performed therapy have been improved in recent years, the prognosis to determine the effect of cisplatin (DDP) after inhibiting the for this disease remains poor.