Feeding Adaptations in Whistling Ducks (Dendroc ¾Gna )
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A 2010 Supplement to Ducks, Geese, and Swans of the World
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 20. https://digitalcommons.unl.edu/biosciducksgeeseswans/20 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The World’s Waterfowl in the 21st Century: A 200 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard Pages xvii–xxiii: recent taxonomic changes, I have revised sev- Introduction to the Family Anatidae eral of the range maps to conform with more current information. For these updates I have Since the 978 publication of my Ducks, Geese relied largely on Kear (2005). and Swans of the World hundreds if not thou- Other important waterfowl books published sands of publications on the Anatidae have since 978 and covering the entire waterfowl appeared, making a comprehensive literature family include an identification guide to the supplement and text updating impossible. -

Waterlines-74 National Waterbird Assessment
National waterbird assessment RT Kingsford, JL Porter and SA Halse Waterlines Report Series No. 74, March 2012 Waterlines This paper is part of a series of works commissioned by the National Water Commission on key water issues. This work has been undertaken by the Australian Wetlands and Rivers Centre of the University of New South Wales on behalf of the National Water Commission. © Commonwealth of Australia 2012 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission. Requests and enquiries concerning reproduction and rights should be addressed to the Communications Director, National Water Commission, 95 Northbourne Avenue, Canberra ACT 2600 or email [email protected]. Online/print: ISBN: 978-1-921853-58-6 National waterbird assessment, March 2012 Authors: RT Kingsford, JL Porter and SA Halse Published by the National Water Commission 95 Northbourne Avenue Canberra ACT 2600 Tel: 02 6102 6000 Email: [email protected] Date of publication: March 2012 Cover design by: Angelink Front cover image courtesy of Prof RT Kingsford An appropriate citation for this report is: Kingsford RT, Porter JL and Halse SA 2011, National waterbird assessment, Waterlines report, National Water Commission, Canberra Disclaimer This paper is presented by the National Water Commission for the purpose of informing discussion and does not necessarily reflect the views or opinions of the Commission. Contents Foreword ix Acknowledgements x Executive summary xi 1. Introduction 1 1.1. Background 2 1.2. Project objectives 11 1.3. Report structure 11 2. -

Waterfowl Collection at Slimbridge 1955-56
Annual Report 1954-56 35 WATERFOWL COLLECTION AT SLIMBRIDGE 1955-56 THE BREEDING SEASON 1955 By S. T. Johnstone T h e feature of the breeding season was the striking effect of cold weather on the well-being of the young birds. Frost in February and March may well have reduced considerably the hatchability of the Ne-Ne eggs, all 31 of which were laid during a period when the cold was so extreme that some African Black Duck eggs were split open before they could be collected. The very wet April and May caused flooding of nests and indeed several sitting boxes suffered in this way. This latter occurrence may have had a bearing on the unfortunate rise in the incidence of Aspergillosis. Pathogenic mould was found in a number of fertile eggs that failed to hatch and a relatively large number of goslings succumbed to mycosis. In 1956 the use of sawdust for nest making in the sitting boxes has been discontinued in favour of peat moss impregnated with a fungicide. The two pumping systems installed in the spring of 1954 have enabled us to provide relatively fast-flowing water through the rearing pens. By this means we have get rid of the concentration of water fleas (.Daphnia pulex). This had been the host of Acuaria uncinata, a worm inhabiting the proventriculus and causing wasting and subsequent death. We are pleased to report that not a single case of Acuaria was recorded in 1955. It was a great relief to those concerned with the rearing when cold and wet ceased and the long warm sunny days of June and July appeared as a panacea to all ills save the losses from predators. -

Parasitic Helminths and Arthropods of Fulvous Whistling-Ducks (Dendrocygna Bicolor) in Southern Florida
J. Helminthol. Soc. Wash. 61(1), 1994, pp. 84-88 Parasitic Helminths and Arthropods of Fulvous Whistling-Ducks (Dendrocygna bicolor) in Southern Florida DONALD J. FORRESTER,' JOHN M. KINSELLA,' JAMES W. MERTiNS,2 ROGER D. PRICE,3 AND RICHARD E. TuRNBULL4 5 1 Department of Infectious Diseases, College of Veterinary Medicine, University of Florida, Gainesville, Florida 32610, 2 U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services, National Veterinary Services Laboratories, P.O. Box 844, Ames, Iowa 50010, 1 Department of Entomology, University of Minnesota, St. Paul, Minnesota 55108, and 4 Florida Game and Fresh Water Fish Commission, Okeechobee, Florida 34974 ABSTRACT: Thirty fulvous whistling-ducks (Dendrocygna bicolor) collected during 1984-1985 from the Ever- glades Agricultural Area of southern Florida were examined for parasites. Twenty-eight species were identified and included 8 trematodes, 6 cestodes, 1 nematode, 4 chewing lice, and 9 mites. All parasites except the 4 species of lice and 1 of the mites are new host records for fulvous whistling-ducks. None of the ducks were infected with blood parasites. Every duck was infected with at least 2 species of helminths (mean 4.2; range 2- 8 species). The most common helminths were the trematodes Echinostoma trivolvis and Typhlocoelum cucu- merinum and 2 undescribed cestodes of the genus Diorchis, which occurred in prevalences of 67, 63, 50, and 50%, respectively. Only 1 duck was free of parasitic arthropods; each of the other 29 ducks was infested with at least 3 species of arthropods (mean 5.3; range 3-9 species). The most common arthropods included an undescribed feather mite (Ingrassia sp.) and the chewing louse Holomenopon leucoxanthum, both of which occurred in 97% of the ducks. -

2.9 Waterbirds: Identification, Rehabilitation and Management
Chapter 2.9 — Freshwater birds: identification, rehabilitation and management• 193 2.9 Waterbirds: identification, rehabilitation and management Phil Straw Avifauna Research & Services Australia Abstract All waterbirds and other bird species associated with wetlands, are described including how habitats are used at ephemeral and permanent wetlands in the south east of Australia. Wetland habitat has declined substantially since European settlement. Although no waterbird species have gone extinct as a result some are now listed as endangered. Reedbeds are taken as an example of how wetlands can be managed. Chapter 2.9 — Freshwater birds: identification, rehabilitation and management• 194 Introduction such as farm dams and ponds. In contrast, the Great-crested Grebe is usually associated with large Australia has a unique suite of waterbirds, lakes and deep reservoirs. many of which are endemic to this, the driest inhabited continent on earth, or to the Australasian The legs of grebes are set far back on the body region with Australia being the main stronghold making them very efficient swimmers. They forage for the species. Despite extensive losses of almost completely underwater pursuing fish and wetlands across the continent since European aquatic arthropods such as insects and crustaceans. settlement no extinctions of waterbirds have They are strong fliers but are poor at manoeuvering been recorded from the Australian mainland as in flight and generally prefer to dive underwater a consequence. However, there have been some to escape avian predators or when disturbed by dramatic declines in many populations and several humans. Flights between wetlands, some times species are now listed as threatened including over great distances, are carried out under the cover the Australasian Bittern, Botaurus poiciloptilus of darkness when it is safe from attack by most (nationally endangered). -

A Preliminary Risk Assessment of Cane Toads in Kakadu National Park Scientist Report 164, Supervising Scientist, Darwin NT
supervising scientist 164 report A preliminary risk assessment of cane toads in Kakadu National Park RA van Dam, DJ Walden & GW Begg supervising scientist national centre for tropical wetland research This report has been prepared by staff of the Environmental Research Institute of the Supervising Scientist (eriss) as part of our commitment to the National Centre for Tropical Wetland Research Rick A van Dam Environmental Research Institute of the Supervising Scientist, Locked Bag 2, Jabiru NT 0886, Australia (Present address: Sinclair Knight Merz, 100 Christie St, St Leonards NSW 2065, Australia) David J Walden Environmental Research Institute of the Supervising Scientist, GPO Box 461, Darwin NT 0801, Australia George W Begg Environmental Research Institute of the Supervising Scientist, GPO Box 461, Darwin NT 0801, Australia This report should be cited as follows: van Dam RA, Walden DJ & Begg GW 2002 A preliminary risk assessment of cane toads in Kakadu National Park Scientist Report 164, Supervising Scientist, Darwin NT The Supervising Scientist is part of Environment Australia, the environmental program of the Commonwealth Department of Environment and Heritage © Commonwealth of Australia 2002 Supervising Scientist Environment Australia GPO Box 461, Darwin NT 0801 Australia ISSN 1325-1554 ISBN 0 642 24370 0 This work is copyright Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Supervising Scientist Requests and inquiries concerning reproduction -

A Partial Classification of Waterfowl (Anatidae) Based on Single-Copy Dna
A PARTIAL CLASSIFICATION OF WATERFOWL (ANATIDAE) BASED ON SINGLE-COPY DNA CORT S. MADSEN, KEVIN P. MCHUGH, AND SIWO R. DE KLOET Instituteof MolecularBiophysics, The Florida State University, Tallahassee, Florida 32306 USA ABSTR^CT.--Single-copyDNA-DNA hybridizationwas used to establishphylogenetic re- lationshipsamong 13 speciesof waterfowl (Anatidae) chosenfrom 10 tribes. On the basisof UPGMA clusteringof ATtodistances, we suggestthat the tribes Anatini, Aythyini, Tadornini, Mergini, and Cairinini diverged more recently than the Anserini, Stictonettini,Oxyurini, Dendrocygnini,and Anseranatini.The FreckledDuck (Stictonettanaevosa, tribe Stictonettini) is only distantlyrelated to the other Anatidae.Presumably the lineage divergedvery early. The sheldgeese(tribe Tadornini) and the true geese(Anserini) are only remotely related. The Oxyurini, consideredto be in the subfamilyAnatinae, is remotely related to the other Anatidae. The Dendrocygnini form an isolatedtribe with no closerelationship to the swans and geese(subfamily Anserinae). We found that the screamers(Anhimidae) are distantly related to the Anatidae. A method to estimate missing cells in a data matrix of pairwise distancesis presented. ReceivedI May 1987,accepted 16 February1988. RECENTLY,nucleic acid sequenceanalysis has up about 10-40% of the total DNA (Eden et al. been usedto estimaterelationships of many or- 1978) but containsonly a small percentageof ganisms. The analysis can include direct se- the total sequencediversity. The remaining60- quencing of individual cloned genes -

Waterfowl of North America: WHISTLING DUCKS Tribe Dendrocygnini
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Waterfowl of North America, Revised Edition (2010) Papers in the Biological Sciences 2010 Waterfowl of North America: WHISTLING DUCKS Tribe Dendrocygnini Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciwaterfowlna Part of the Ornithology Commons Johnsgard, Paul A., "Waterfowl of North America: WHISTLING DUCKS Tribe Dendrocygnini" (2010). Waterfowl of North America, Revised Edition (2010). 8. https://digitalcommons.unl.edu/biosciwaterfowlna/8 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Waterfowl of North America, Revised Edition (2010) by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. WHISTLING DUCKS Tribe Dendrocygnini Whistling ducks comprise a group of nine species that are primarily of tropical and subtropical distribution. In common with the swans and true geese (which with them comprise the subfamily Anserinae), the included spe cies have a reticulated tarsal surface pattern, lack sexual dimorphism in plum age, produce vocalizations that are similar or identical in both sexes, form relatively permanent pair bonds, and lack complex pair-forming behavior pat terns. Unlike the geese and swans, whistling ducks have clear, often melodious whistling voices that are the basis for their group name. The alternative name, tree ducks, is far less appropriate, since few of the species regularly perch or nest in trees. All the species have relatively long legs and large feet that extend beyond the fairly short tail when the birds are in flight. -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
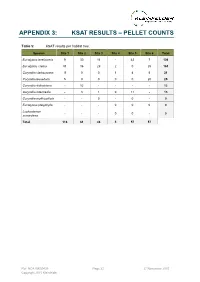
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential -

Differential Use of Fresh Water Environments by Wintering Waterfowl of Coastal Texas
DIFFERENTIAL USE OF FRESH WATER ENVIRONMENTS BY WINTERING WATERFOWL OF COASTAL TEXAS DONALD H. WHITE AND DOUGLAS JAMES Species having similar life styles (Ralph 1975) characteristically occupy different ecological niches (Hutchinson 1957, 1965) within shared environ- ments. Many workers have shown that this principle seems to be operative in avian communities (MacArthur 1958, Cody 1968, James 1971, Posey 1974, Whitmore 1975). Our study was conducted to determine how feeding flocks of wintering waterfowl coexisted in feeding site selection, what environmental factors that were measured were the most important in certain aspects of niche separation, and how the niches were arranged in the aquatic community at the study site. METHODS AND MATERIALS Study area.-Data were collected from early October through late December 1973 from 2 adjacent ox-bow lakes on the grounds of the Welder Wildlife Foundation in San Patricia County near Sinton, Texas. These fresh water lakes were up to 2.5 m deep but averaged about 1.5 m in the middle. A broad zone of semi-aquatic grasses (Paspolum and Panicurn) occupied the perimeters and burhead (Echinodorus rostratus), southern cut- grass (Lee&a hexandra), and bulrush (Scirpus californicus) occurred in isolated small patches. The transition zone from emergent semi-aquatic vegetation sometimes occurred over 90 m from shore, but was quite variable in position. Extensive floating or partly sub- merged patches of aquatic vegetation were dominated by southern naiad (Noios guadalupensis), star grass (Heteranthera liebmannii), musk grass (Chara), and duck weed (Lemna perpusilla) . Large numbers of waterfowl use the coastal region of southern Texas during the fall and winter months (Bellrose 1976) therefore, references to “wintering waterfowl” and “wintering grounds” throughout this paper are made on this basis. -

The Rice Agroecosystem, Cuban Fulvous Whistling Ducks and Avian Conservation
THE RICE AGROECOSYSTEM, CUBAN FULVOUS WHISTLING DUCKS AND AVIAN CONSERVATION Lourdes Mugica Valdes Lic., Universidad de la Habana 1 981 r THESIS SUBMllTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCES in the Department of Biological Sciences O Lourdes Mugica Valdes 1993 SIMON FRASER UNIVERSITY October 1993 All rights resewed. This work may not be reproduced in whole or in part, by photocopy or other means, without permission of the author. APPROVAL Name: LOURDES MUGICA-VALDES Degree: Master of Science f Title of Thesis: THE RICE AGROECOSYSTEM, CUBAN FULVOUS WHISTLING DUCKS, AND AVIAN CONSERVATION Examining Committee: Chair: Dr. N.H. Haunerland, Associate Professor Senior Supervisor, Departme Dr. N.Af ~er&k;fiofEssor, Depart ent of Biological Sciences, SFU Dr. Kee Bass, AssociaYe Piofebsor Department of Zoology, UBC External Examiner Date Approved 5-8 - /.9?3 . PART I AL COPYR 1 GHT L l CENSE I hereby grant to Simon Fraser Unlverslty the right to lend my thesis, project or extended essay'(the ,ltle of whlch Is shown below) to users of the Slmh Frarer Unlversl ty ~lbr&~, and to make part la l or single coples only for such users or In response to a request from the 1 i brary of any other unlverslty, or other educat lona l I nst I tut Ion, on its own behalf or for one of Its users. I further agree that permission formultlple copying of thls work for scholarly purposes may be granted by me or the Dean of Graduate Studles. It is understood that copyrng or publlcatlon of thls work for flnanclal gain shall not be allowed without my written permlsslon. -

Ducks, Geese, and Swans of the World by Paul A
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 Ducks, Geese, and Swans of the World: Contents, Preface, & Introduction Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "Ducks, Geese, and Swans of the World: Contents, Preface, & Introduction" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 2. https://digitalcommons.unl.edu/biosciducksgeeseswans/2 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. DUCKS, GEESE, and SWANS of the World Paul A. Johnsgard Revised Edition Ducks, Geese, and Swans of the World By Paul A. Johnsgard The only one-volume comprehensive survey of the family Anatidae available in English, this book combines lavish illustration with the most recent information on the natural history, current distribution and status, and identification of all the species. After an introductory discussion of the ten tribes of Anatidae, separate accounts follow for each of the nearly 150 recognized species. These include scientific and vernacular names (in French, German, and Spanish as well as English), descrip- tions of the distribution of all recognized subspecies, selected weights and mea- surements, and identification criteria for both sexes and various age classes.