Robustness of Biological and Bio-Inspired Exoskeletons By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Power of Talent
Black Red CharcoalGray WarmGray Power of talent “Our core corporate assets walk out every evening. It is our duty to make sure that these assets return the next morning, mentally and physically enthusiastic and energetic.” – N. R. Narayana Murthy, Chairman and Chief Mentor This Annual Report is printed on 100% recycled paper as certified by the UK-based National Association of Paper Merchants (NAPM). The paper complies with strict quality and environmental requirements and is awarded the following international eco-labels: the Nordic Swan from the Nordic Council of Ministers; the Blue Angel, the first worldwide environmental label awarded by the Jury Umweltzeichen; and the European Union Flower. Infosys Annual Report 2007-08 | 1 Eugenie Rosenthal InStep intern in 2007 Mardava Rajugopal Catch Them Young participant in 2007 Deepak Gopinath Catch Them Young participant in 2007 Connect In 2007, an impressive 66% of 350 colleges surveyed opined that our Campus Connect program has helped make their students more employable. 2 | Power of talent Black Red CharcoalGray WarmGray Development Center, Bangalore, India By 2010, the Indian IT industry will need significantly contributed to our cultural “CTY was an enriching 2.3 million software engineers, says a and intellectual diversity. In the last nine experience. The courses were NASSCOM study. The estimated shortfall years, we have had over 600 interns from conducted in an encouraging, is 22%. This shortfall is one of the key 85 premier universities such as Harvard, open atmosphere which helped reasons we think it is imperative to connect Stanford, MIT and Oxford. InStep has us think out of the box and make the academic world with the industry. -

Masculinity and the Structuring of the Public Domain in Kerala: a History of the Contemporary
MASCULINITY AND THE STRUCTURING OF THE PUBLIC DOMAIN IN KERALA: A HISTORY OF THE CONTEMPORARY Ph. D. Thesis submitted to MANIPAL ACADEMY OF HIGHER EDUCATION (MAHE – Deemed University) RATHEESH RADHAKRISHNAN CENTRE FOR THE STUDY OF CULTURE AND SOCIETY (Affiliated to MAHE- Deemed University) BANGALORE- 560011 JULY 2006 To my parents KM Rajalakshmy and M Radhakrishnan For the spirit of reason and freedom I was introduced to… This work is dedicated…. The object was to learn to what extent the effort to think one’s own history can free thought from what it silently thinks, so enable it to think differently. Michel Foucault. 1985/1990. The Use of Pleasure: The History of Sexuality Vol. II, trans. Robert Hurley. New York: Vintage: 9. … in order to problematise our inherited categories and perspectives on gender meanings, might not men’s experiences of gender – in relation to themselves, their bodies, to socially constructed representations, and to others (men and women) – be a potentially subversive way to begin? […]. Of course the risks are very high, namely, of being misunderstood both by the common sense of the dominant order and by a politically correct feminism. But, then, welcome to the margins! Mary E. John. 2002. “Responses”. From the Margins (February 2002): 247. The peacock has his plumes The cock his comb The lion his mane And the man his moustache. Tell me O Evolution! Is masculinity Only clothes and ornaments That in time becomes the body? PN Gopikrishnan. 2003. “Parayu Parinaamame!” (Tell me O Evolution!). Reprinted in Madiyanmarude Manifesto (Manifesto of the Lazy, 2006). Thrissur: Current Books: 78. -

Conference Program July 26-29, 2021 | Pacific Daylight Time 2021 Asee Virtual Conference President’S Welcome
CONFERENCE PROGRAM JULY 26-29, 2021 | PACIFIC DAYLIGHT TIME 2021 ASEE VIRTUAL CONFERENCE PRESIDENT’S WELCOME SMALL SCREEN, SAME BOLD IDEAS It is my honor, as ASEE President, to welcome you to the 128th ASEE Annual Conference. This will be our second and, almost certainly, final virtual conference. While we know there are limits to a virtual platform, by now we’ve learned to navigate online events to make the most of our experience. Last year’s ASEE Annual Conference was a success by almost any measure, and all of us—ASEE staff, leaders, volunteers, and you, our attendees—contributed to a great meeting. We are confident that this year’s event will be even better. Whether attending in person or on a computer, one thing remains the same, and that’s the tremendous amount of great content that ASEE’s Annual Conference unfailingly delivers. From our fantastic plenary speakers, paper presentations, and technical sessions to our inspiring lineup of Distinguished Lectures and panel discussions, you will have many learning opportunities and take-aways. I hope you enjoy this week’s events and please feel free to “find” me and reach out with any questions or comments! Sincerely, SHERYL SORBY ASEE President 2020-2021 2 Schedule subject to change. Please go to https://2021asee.pathable.co/ for up-to-date information. 2021 ASEE VIRTUAL CONFERENCE TABLE OF CONTENTS 2021 ASEE VIRTUAL CONFERENCE AND EXPOSITION PROGRAM ASEE BOARD OF DIRECTORS ................................................................................4 CONFERENCE-AT-A-GLANCE ................................................................................6 -

Message from the TMB President: COVID-19 Agency Update
www.tmb.state.tx.us Follow TMB on Facebook October 2020 Message From the TMB President: COVID - 19 Agency Update During the most recent Board We encourage licensees to share information and re- meeting in August, we provided an sources with your colleagues, continue seeking help and update on the Board’s ongoing assistance from your local medical societies and profes- efforts during the COVID-19 pandem- sional workplace groups, and please continue checking in ic. Since the onset of the pandemic, with co-workers and supporting one another. Board staff have issued over 2,800 temporary emergency licenses and The Board will continue to do everything it can to ensure the health and safety of Texans, including our licensees. Zaafran reactivated 60 licenses for recently retired health care professionals. Though the situation in Texas is markedly improved from TMB staff has managed to process these licenses in a where we were several months ago, we should all con- matter of just a few days. tinue to be diligent in following the recommended public health guidelines. It’s worth noting that all of this work is being accom- plished in addition to the already steady workload of This will not only help your family, friends and neighbors processing thousands of regular licensure applicants to in staying healthy and safe, but also help our fellow ensure our state continues to have a strong health care frontline health care professionals as they continue their workforce ready to help care for our fellow Texans dur- hard work caring for our most critical patients. -

Twenty 20 Malayalam Full Movie
Twenty 20 malayalam full movie Continue Twenty:20Official theatrical posterFoshiProduced byDileep Written byUdayakrishnaSiby K. ThomasStarringMohanlalMammoottySuresh GopiJayaramDileepMusic fromSur PetereshsBerny IgnatiusCinematographyP.SukumarEditedRan by Abraham Abraham and production DistributedManjunatha release Kalasangham FilmsRelease date November 5, 2008 (2008-11-05) Duration 165 minutesCountryIndiaLanguageMalayalamBudget₹7 crore (US$980,000)Box officeest. ₹32.6 crora ($4.6 million) is a 2008 Indian action film in Malayalam, written by Udayakrishna and Sibi K. Thomas and directed by Josh. The film stars Mohanlal, Mammutti, Suresh Gopi, Jayaram and Dillep, and was produced and distributed by actor Dillep through Graand Production and Manjunatha Release. The film was produced on behalf of the Association of Malayalam Film Chronicles (AMMA) as a fundraiser to financially support actors who are struggling in the Malayalam film industry. All AMMA members worked without pay to raise funds for their Social Security programs. The film features an ensemble of actors, which includes almost all AMMA artists. The music was written by Bernie-Ignatius and Suresh Peters. The share of the first two weeks of the film's distributors was ₹5.72 crore ($800,000). The film managed to gain a foothold in the third position (with 7 prints, 4 in Chennai) in the box office of Tamil Nadu in the first week. The total number of introductory engravings produced inside Kerala was 117; some 25 prints were released outside Kerala on November 21, 2008, including 4 prints in the United States and 11 engravings in the UAE. The film grossed a total of ₹32.6 kronor ($4.6 million). It was the highest-grossing film in Malayalam cinema until 2013. -

Mohanlal Filmography
Mohanlal filmography Mohanlal Viswanathan Nair (born May 21, 1960) is a four-time National Award-winning Indian actor, producer, singer and story writer who mainly works in Malayalam films, a part of Indian cinema. The following is a list of films in which he has played a role. 1970s [edit]1978 No Film Co-stars Director Role Other notes [1] 1 Thiranottam Sasi Kumar Ashok Kumar Kuttappan Released in one center. 2 Rantu Janmam Nagavally R. S. Kurup [edit]1980s [edit]1980 No Film Co-stars Director Role Other notes 1 Manjil Virinja Pookkal Poornima Jayaram Fazil Narendran Mohanlal portrays the antagonist [edit]1981 No Film Co-stars Director Role Other notes 1 Sanchari Prem Nazir, Jayan Boban Kunchacko Dr. Sekhar Antagonist 2 Thakilu Kottampuram Prem Nazir, Sukumaran Balu Kiriyath (Mohanlal) 3 Dhanya Jayan, Kunchacko Boban Fazil Mohanlal 4 Attimari Sukumaran Sasi Kumar Shan 5 Thenum Vayambum Prem Nazir Ashok Kumar Varma 6 Ahimsa Ratheesh, Mammootty I V Sasi Mohan [edit]1982 No Film Co-stars Director Role Other notes 1 Madrasile Mon Ravikumar Radhakrishnan Mohan Lal 2 Football Radhakrishnan (Guest Role) 3 Jambulingam Prem Nazir Sasikumar (as Mohanlal) 4 Kelkkatha Shabdam Balachandra Menon Balachandra Menon Babu 5 Padayottam Mammootty, Prem Nazir Jijo Kannan 6 Enikkum Oru Divasam Adoor Bhasi Sreekumaran Thambi (as Mohanlal) 7 Pooviriyum Pulari Mammootty, Shankar G.Premkumar (as Mohanlal) 8 Aakrosham Prem Nazir A. B. Raj Mohanachandran 9 Sree Ayyappanum Vavarum Prem Nazir Suresh Mohanlal 10 Enthino Pookkunna Pookkal Mammootty, Ratheesh Gopinath Babu Surendran 11 Sindoora Sandhyakku Mounam Ratheesh, Laxmi I V Sasi Kishor 12 Ente Mohangal Poovaninju Shankar, Menaka Bhadran Vinu 13 Njanonnu Parayatte K. -

Screening the Impossible: the Politics of Form and Feeling in Second Wave Revolutionary Cinema
SCREENING THE IMPOSSIBLE: THE POLITICS OF FORM AND FEELING IN SECOND WAVE REVOLUTIONARY CINEMA By Sarah Hamblin A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY English 2012 ABSTRACT SCREENING THE IMPOSSIBLE: THE POLITICS OF FORM AND FEELING IN SECOND WAVE REVOLUTIONARY CINEMA By Sarah Hamblin Screening the Impossible explores how the new revolutionary ideologies that emerged in the various global articulations of the “long 1968” produced new forms of revolutionary cinematic practice – what I collectively refer to as a second wave of revolutionary filmmaking. The project focuses on films largely from the 1960s and 1970s that engage the revolutionary energies of the period to examine the relationship between emotion, aesthetics, and political theory in an international cinematic context. Drawing on the claim that the global rebellions of the 1960s mark the denunciation of early 20th century revolutionary narratives, it traces the connections between filmmakers who are similarly preoccupied with the limits, failures, and counter-revolutionary appropriations of orthodox revolutionary thought and yet remain committed to the necessity of revolutionary transformation. Through a comparative analysis of films from various national traditions, the project examines how the political cinema of this period develops a new understanding of revolutionary process and the role that cinema can play in it. At its core, the project lays out the aesthetic and affective contours of this emergent genre, arguing that second wave revolutionary cinema is characterized by its rejection of the teleological narratives and didactic political messages embedded in earlier first wave revolutionary cinematic production. -

Accused Persons Arrested in Thrissur City District from 17.09.2017 to 23.09.2017
Accused Persons arrested in Thrissur City district from 17.09.2017 to 23.09.2017 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 MEDICAL P. U 25, VADAKOOT, MUNDATHI 17-09-2017 702/2017 U/s COLLEGE BAILED BY 1 GINEESH GOPI SETHUMAD Male VELAPAYA KODE at 13:00 279 IPC (THRISSUR POLICE HAVAN CITY) MEDICAL CHITALAPPILLY, 703/2017 U/s P. U 35, 17-09-2017 COLLEGE BAILED BY 2 ANTONY FRANCIS PUTHENMADAM I/F MCH 279 IPC & 185 SETHUMAD Male at 14:40 (THRISSUR POLICE KUNNU, THIROOR MV ACT HAVAN CITY) 904/2017 U/s GURUVAY THOTTUPURATH SI 32, KOTTAPPAD 17-09-2017 279 IPC & 185 UR BAILED BY 3 AVINASH RAJAN H,ANAKKARA PO, VANILKUM Male Y at 14:00 & 3(1) r/w (THRISSUR POLICE PALAKKAD AR 181 MV Act CITY) THAIKKANATH PERAMAN SHIJU.S.S JR HOUSE, 940/2017 U/s 48, 17-09-2017 GALAM SI BAILED BY 4 ROY LONAPPAN AMBAKKAD AMBAKKAD 15(c) r/w 63 Male at 16:35 (THRISSUR PERAMANG POLICE DESOM. of Abkari Act CITY) ALAM PUZHAKKAL PANENGADAN PERAMAN SHIJU.S.S JR HOUSE, 940/2017 U/s 47, 17-09-2017 GALAM SI BAILED BY 5 SONY PAUL PAUL AMBAKKAD AMBAKKAD 15(c) r/w 63 Male at 16:35 (THRISSUR PERAMANG POLICE DESOM of Abkari Act CITY) ALAM PUZHAKKAL S/O 963/2017 U/s PEECHI P. -
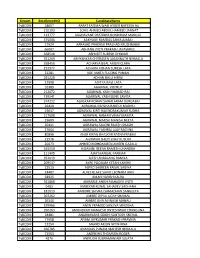
Stream Enrollmentno Candidatename Tybcom 18657
Stream EnrollmentNO CandidateName TyBCOM 18657 . RAFAT FATIMA WASI HYDER NAFEESA ALI TyBCOM 210193 . SOHIL AHMED ABDUL HAMEED JANNAT TyBCOM 143777 AAGHAVANE PRATIBHA KONDIRAM MANGAL TyBCOM 375006 AAKHADE RAMDAS SAMA JIJABAI TyBCOM 17924 AARASAD PRAKASH PRALHAD KRUSHNABAI TyBCOM 66007 ABHANG JYOTI PRAKASH JAYASHREE TyBCOM 368518 ABHIJEET SURESH DHIWAR TyBCOM 351269 ABHYANKAR CHITRASEN JAGANNATH NIRMALA TyBCOM 358456 ACHARYA BIJAL ARJUN CLARA TyBCOM 152977 ACHHRA KISHAN SURESH LATA TyBCOM 22281 ADE AMITA FULSING PANBAI TyBCOM 261226 ADHAN BALU HIRAJI TyBCOM 27998 ADITYA RAVI LATA TyBCOM 16389 AGARWAL VISHRUT TyBCOM 212072 AGARWAL YASH SHARAD RAJ TyBCOM 139541 AGARWAL YASH SUNIL SAVITA TyBCOM 374212 AGASKAR ROHAN SAWALARAM DURGABAI TyBCOM 26429 AGRAWAL DIVISHA RAKESH MADHU TyBCOM 19637 AGRAWAL KIRTI RAJENDRAKUMAR SUDHA TyBCOM 217608 AGRAWAL NAMAN VINAY MAMTA TyBCOM 29689 AGRAWAL NIMISH MANISH NEETA TyBCOM 20441 AGRAWAL SALONI RAJESH SHASHI TyBCOM 27664 AGRAWAL YASHRAJ AJAY RACHNA TyBCOM 80896 AHER KIRAN BHASKAR RATNAPRABHA TyBCOM 21576 AHIRWAR SHILPI VIJAY KUSUM TyBCOM 20073 AHMED MOHDAMEEN AMEEN GAZALA TyBCOM 345550 AIDASANI REENA RAMESH CHANDRA TyBCOM 111495 AJAY MANGAL PARMAR TyBCOM 352019 AJESH ANBALANG RAMILA TyBCOM 209537 AKRE POONAM KETAN KAMINI TyBCOM 22513 ALPRO SABREEN FAISAL SABIHA TyBCOM 24487 ALVES BLAKE SAVIO LEONARA ANN TyBCOM 58925 AMAN HARISH KALRA TyBCOM 351868 AMBARLE ANISH MAHADEV JYOTI TyBCOM 9483 AMBEKAR KUNAL SAHADEV SADHANA TyBCOM 181019 AMBORE SHIVRAJ SAMADHAN SANGEETA TyBCOM 307262 AMBRE DIPALI UDAY SHAMAL TyBCOM -
Participant List
Participant List 4/14/2021 7:38:33 PM Category First Name Last Name Position Organization Nationality CSO Babak Abbaszadeh President And Chief Toronto Centre For Global Canada Executive Officer Leadership In Financial Supervision Ziad Abdel Samad Executive Director Arab NGO Network for Lebanon Development Tazi Abdelilah Président Associaion Talassemtane pour Morocco l'environnement et le développement ATED Dr. Ghada Abdelsalam Senior Tax manager Egyptian Tax Authority Egypt Ziad ABDELTAWA Deputy Director Cairo institute for Human Egypt B Rights Studies (CIHRS) Sadak Abdi Fishery Development Hifcon Somalia Nabil Abdo MENA Senior Policy Oxfam International Lebanon Advisor Maryati Abdullah Director/National Publish What You Pay Indonesia Coordinator Indonesia Diam Abou Diab Senior program and Arab NGO Network for Lebanon research officer Development Hayk Abrahamyan Community Organizer for International Accountability Armenia South Caucasus and Project Central Asia Barbara Adams Board Chair Global Policy Forum Canada Ben Adams Senior Advisor Mental CBM Global Ireland Health Abiodun Aderibigbe Head of Research and sustainable Environment Food Nigeria project Development and Agriculture Initiative Bamisope Adeyanju Policy Fellow Accountability Counsel Nigeria Mange Adhana President Association For Promotion India Sustainable development Ezatullah Adib Head of Research Integrity Watch Afghanistan Afghanistan Mirna Adjami Program Manager DCAF - Geneva Centre for Switzerland Security Sector Governance Tity Agbahey Africa Regional Coordinator Coalition -

We Tv - Feature Film Schedule for September 2019 Name of the Film Day Time
WE TV - FEATURE FILM SCHEDULE FOR SEPTEMBER 2019 NAME OF THE FILM DAY TIME DATE 01.09.2019 Loham Sunday 09.00AM 01.09.2019 Pathemaari SundayAN 02.30PM 01.09.2019 Oru Vadakkan Selfi SundayNIGHT08.30PM 02.09.2019 Thuruppugulan Monday 09.00AM 02.09.2019 Amma Ammayiamma MondayAN 02.30PM 02.09.2019 Jilla (Dubbed) MondayNIGHT08.30PM 03.09.2019 Vandan vendran (Dubbed) Tuesday 09.00AM 03.09.2019 Singham II (Dubbed) TuesdayAN 02.30PM 03.09.2019 Premam TuesdayNIGHT08.30PM 04.09.2019 Oppam Wednesday 09.00AM 04.09.2019 Kudumbasametham WednesdayAN02.30PM 04.09.2019 Malarvadi Arts Club WednesdayNI0G8H.3T0PM 05.09.2019 Njan Salperu Ramankutty Thursday 09.00AM 05.09.2019 Oppam Oppathinoppam ThursdayAN 02.30PM 05.09.2019 Rajini Murugan (Dubbed) ThursdayNIGH0T8.30PM 06.09.2019 Anjaan (Dubbed) Friday 09.00AM 06.09.2019 Druvangal 16 (Dubbed) FridayAN 02.30PM 06.09.2019 SethuPathi (Dubbed) FridayNIGHT 08.30PM 07.09.2019 Ji (dubbed) Saturday 09.00AM 07.09.2019 Varalaru (Dubbed) SaturdayAN 02.30PM 07.09.2019 Red (Dubbed) SaturdayNIGH0T8.30PM 08.09.2019 Anjaneya (Dubbed) Sunday 09.00AM 08.09.2019 Kakkakuyil SundayAN 02.30PM 08.09.2019 Puli (Dubbed) SundayEVE 05.30PM 08.09.2019 Ustaad SundayNIGHT08.30PM 09.09.2019 Kaashmora (Dubbed) Monday 09.00AM 09.09.2019 Mersal (Dubbed) MondayAN 02.30PM 09.09.2019 Crazy Gopalan MondayEVE 05.30PM 09.09.2019 KO 2 (Dubbed) MondayNIGHT08.30PM 10.09.2019 Thuppakki (Dubbed) Tuesday 09.00AM 10.09.2019 Rajanimurugan TuesdayAN 02.30PM 10.09.2019 Vedhalam (Dubbed) TuesdayEVE 05.30PM 10.09.2019 Kaththi (Dubbed) TuesdayNIGHT08.30PM 11.09.2019 -

56 National Film Awards
56th NATIONAL FILM AWARDS FEATURE FILMS JURY Chairman Shri Shaji N. Karun Members Shri Roshan Taneja Shri H.M. Ramachandra Ms Nagma Shri Satyabrata Kalita Shri Neelakanta Shri Dilip Ghosh Shri Swapan Mullick Shri Sudesh Syal Shri S.K. Srivastava Ms Archana Shri B. Shashi Kumar Shri Subhash Sehgal Shri Santosh Desai Ms Sreelekha Mukherjee 56th NATIONAL FILM AWARDS NON-FEATURE FILMS JURY Chairperson Ms Arunaraje Patil Members Shri Krishnendu Bose Shri Anirban Dutta Shri Sandeep Marwah Ms Reena Mohan Shri R. V. Ramani Shri Sarfaraz Siddiqui JURY FOR BEST WRITING ON CINEMA Chairman Shri Sunil Gangopadhyay Members Shri Sudhish Pachauri Shri Zia-us Salam 56TH NATIONAL FILM AWARDS ENTRIES NON-FEATURE FILMS S.No Title Language Director Format 1. And the Friends of Assamese Kaju Video Kaziranga 2. Boliya Pitaier Assamese & Altaf Mazid Video Sohoki Sootal Bodo 3. Baba Black Beard Bengali Sanghamitra Karmakar Video 4. Fables of Birth Bengali & Sahana Bhattacharya Video Santhali 5. I’m the Very Bengali Shyamal Kumar Video Beautiful Karmakar 6. Jibaner Jalsaghorey Bengali Satarupa Sanyal Video 7. Kalo Sheikh Bengali AlokeDas Video 8. Padma Patar Jal Bengali Dhananjay Mandal Video 9. Shabnam Bengali /EST Aditi Sircar Video 10. The Shop That Sold Bengali Abhyuday Khaitan 35mm Everything 11. Songs, Colours and Bengali Joshy Joseph 35mm Market 12. Yearn to Learn- Bengali S.K.Abdul Rajjak Video Madrasah Education in West Bengal 13. The Assassination of English R.Krishna Mohan 35mm Rajiv Gandhi-A reconstruction 14. Blood on My Hands English Manak Matiyani Video 15. Broken Wings-The English Mike H.Pandey Video Vanishing Cultures 16.