Tropical Agricultural Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

The World Lobster Market
GLOBEFISH RESEARCH PROGRAMME The world lobster market Volume 123 GRP123coverB5.indd 1 23/01/2017 15:06:37 FAO GLOBEFISH RESEARCH PROGRAMME VOL. 123 The world lobster market by Graciela Pereira Helga Josupeit FAO Consultants Products, Trade and Marketing Branch Fisheries and Aquaculture Policy and Resources Division Rome, Italy FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2017 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-109631-4 © FAO, 2017 FAO encourages the use, reproduction and dissemination of material in this information product. Except where otherwise indicated, material may be copied, downloaded and printed for private study, research and teaching purposes, or for use in non-commercial products or services, provided that appropriate acknowledgement of FAO as the source and copyright holder is given and that FAO’s endorsement of users’ views, products or services is not implied in any way. -
Lobsters LOBSTERS§
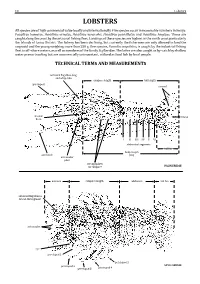
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Florida Spiny Lobster Glazing Florida Lobster
Seafood Safe Handling Tips Florida Spiny Lobster Glazing Florida Lobster Spiny lobster (Panulirus argus) is a crustacean related Frozen lobster is “glazed” with a thin coat of ice Purchase seafood last and keep it cold during the to crabs, shrimp, crayfish and the Spanish lobster. and packaged in plastic to protect the meat from trip home. Spiny lobster has numerous spines on the body, two dehydration and freezer burn. The net weight listed Keep raw and cooked seafood separate to prevent large hooked horns over the eyes, a pair of long, jointed on the packaging must be the “unglazed” weight of the bacterial cross-contamination. antennae and five pairs of walking legs but no claws. product. For weighing purposes, the product should The shell on the body and tail has mottled coloring of After handling raw seafood, thoroughly wash knives, be rinsed only long enough to remove the glaze. If the yellow, brown, orange and blue markings but it turns a cutting surfaces, sponges and hands with hot soapy glaze is excessive and you are charged lobster price for bright red-orange when the lobster is cooked. Florida water. excess ice, it is mislabeled. spiny lobster is commercially harvested off the southern tip of Florida and the Florida Keys. It is Mislabeling seafood is illegal. If you believe a seafood Buying and Storing Tips caught live using special traps set at depths of 6 to 300 product purchased from a seafood retail store or feet. Its diet consists of clams, snails, seaweed and small supermarket seafood counter is mislabeled, please Live lobster should have some leg movement marine organisms. -

SARAWAK GOVERNMENT GAZETTE PART II Published by Authority
For Reference Only T H E SARAWAK GOVERNMENT GAZETTE PART II Published by Authority Vol. LXXI 25th July, 2016 No. 50 Swk. L. N. 204 THE ADMINISTRATIVE AREAS ORDINANCE THE ADMINISTRATIVE AREAS ORDER, 2016 (Made under section 3) In exercise of the powers conferred upon the Majlis Mesyuarat Kerajaan Negeri by section 3 of the Administrative Areas Ordinance [Cap. 34], the following Order has been made: Citation and commencement 1. This Order may be cited as the Administrative Areas Order, 2016, and shall be deemed to have come into force on the 1st day of August, 2015. Administrative Areas 2. Sarawak is divided into the divisions, districts and sub-districts specified and described in the Schedule. Revocation 3. The Administrative Areas Order, 2015 [Swk. L.N. 366/2015] is hereby revokedSarawak. Lawnet For Reference Only 26 SCHEDULE ADMINISTRATIVE AREAS KUCHING DIVISION (1) Kuching Division Area (Area=4,195 km² approximately) Commencing from a point on the coast approximately midway between Sungai Tambir Hulu and Sungai Tambir Haji Untong; thence bearing approximately 260º 00′ distance approximately 5.45 kilometres; thence bearing approximately 180º 00′ distance approximately 1.1 kilometres to the junction of Sungai Tanju and Loba Tanju; thence in southeasterly direction along Loba Tanju to its estuary with Batang Samarahan; thence upstream along mid Batang Samarahan for a distance approximately 5.0 kilometres; thence bearing approximately 180º 00′ distance approximately 1.8 kilometres to the midstream of Loba Batu Belat; thence in westerly direction along midstream of Loba Batu Belat to the mouth of Loba Gong; thence in southwesterly direction along the midstream of Loba Gong to a point on its confluence with Sungai Bayor; thence along the midstream of Sungai Bayor going downstream to a point at its confluence with Sungai Kuap; thence upstream along mid Sungai Kuap to a point at its confluence with Sungai Semengoh; thence upstream following the mid Sungai Semengoh to a point at the midstream of Sungai Semengoh and between the middle of survey peg nos. -

A Time Series of California Spiny Lobster (Panulirus Interruptus) Phyllosoma from 1951 to 2008 Links Abundance to Warm Oceanogr
KOSLOW ET AL.: LOBSTER PHYLLOSOMA ABUNDANCE LINKED TO WARM CONDITIONS CalCOFI Rep., Vol. 53, 2012 A TIME SERIES OF CALIFORNIA SPINY LOBSTER (PANULIRUS INTERRUPTUS) PHYLLOSOMA FROM 1951 TO 2008 LINKS ABUNDANCE TO WARM OCEANOGRAPHIC CONDITIONS IN SOUTHERN CALIFORNIA J. ANTHONY KOSLOW LauRA ROGERS-BENNETT DOUGLAS J. NEILSON Scripps Institution of Oceanography California Department of Fish and Game California Department of Fish and Game University of California, S.D. Bodega Marine Laboratory 4949 Viewridge Avenue La Jolla, CA 92093-0218 UC Davis, 2099 Westside Rd. San Diego, CA 92123 ph: (858) 534-7284 Bodega Bay, CA 94923-0247 [email protected] ABSTRACT The California spiny lobster (Panulirus interruptus) population is the basis for a valuable commercial and recreational fishery off southern California, yet little is known about its population dynamics. Studies based on CalCOFI sampling in the 1950s indicated that the abun- dance of phyllosoma larvae may be sensitive to ocean- ographic conditions such as El Niño events. To further study the potential influence of environmental variabil- ity and the fishery on lobster productivity, we developed a 60-year time series of the abundance of lobster phyl- losoma from the historical CalCOFI sample collection. Phyllosoma were removed from the midsummer cruises when the early-stage larvae are most abundant in the plankton nearshore. We found that the abundance of the early-stage phyllosoma displayed considerable inter- annual variability but was significantly positively corre- Figure 1. Commercial (solid circles), recreational (open triangles), and total lated with El Niño events, mean sea-surface temperature, landings (solid line) of spiny lobster off southern California. -

Tender for Construction of Substation Civil Works In
Tender for Construction of Substation Civil Works in Bintulu Region (Bintulu Town, Tatau, Kuala Tatau, Sebauh, Ulu Sebauh, Samalaju, Sg Asap, Bakun, Murum, Belaga and Inclusive All Outstation Areas Under Bintulu Jurisdiction) (TENDER REF. NO.: BTU 2/2021/SS) TENDER NOTICE Suitably qualified tenderers are invited for the Tender for Construction of Substation Civil Works in Bintulu Region (Bintulu Town, Tatau, Kuala Tatau, Sebauh, Ulu Sebauh, Samalaju, Sg Asap, Bakun, Murum, Belaga and Inclusive All Outstation Areas Under Bintulu Jurisdiction) stated as follows: Tender Reference No. Title Eligibility Tender for Construction of Substation Civil Works in • UPK under Works, Class D or above, Head I Sub- Bintulu Region (Bintulu Town, Tatau, Kuala Tatau, Sebauh, Head 1, Head II Sub-Head 2, and BTU 2/2021/SS Ulu Sebauh, Samalaju, Sg Asap, Bakun, Murum, Belaga • CIDB Grade G2 or above, Category CE21 and B04 and Inclusive All Outstation Areas Under Bintulu • past project experience Jurisdiction) Instruction to Tenderers: Mandatory Requirement Tenderers shall possess adequate financial capability and experience as follows: • UPK under Works, Class D or above, Head I Sub-Head 1, Head II Sub-Head 2, and • CIDB Grade G2 or above, Category CE21 and B04 • past project experience *Interested tenderers are required to produce evidence of fulfilling above-mentioned mandatory requirement. Failing which, your intention will be rejected. General Instructions 1. This tender exercise will be conducted on an online Ariba platform. The entire event will be managed by SEPRO (Sarawak Energy e-Procurement) Team. Tender details are available for viewing at https://etender.sarawakenergy.com/etender/notice/notice.jsp 2. -

The Balingian Shear Zone and West Baram Line, Sarawak and Their Importance in the Early Cenozoic Evolution of Nw Borneo Robert B
217 THE BALINGIAN SHEAR ZONE AND WEST BARAM LINE, SARAWAK AND THEIR IMPORTANCE IN THE EARLY CENOZOIC EVOLUTION OF NW BORNEO ROBERT B. TATE Department of Geology, University of Malaya, 59100 Kuala Lumpur Intense shearing of the rocks at the Sibu-Bintulu road bridge site on the Sg. Balingian was recognised first in 1976 and the shaaring then attributed to a NE-trending tectonic feature related to the Mulu Shear Zone (McManus & Tate, 1976). New roadside exposures in the middle-upper Eocene Belaga Formation in the neighbourhood of Sg. Balingian between Sibu and Bintulu now reveal a major zone of deformation which seems to trend approximately WNW. The zone appears to continue offshore where it is aligned with a positive gravity anomaly trending WNW (Hutchison,1991) indicating a major discontinuity at depth. The gravity anomaly coincides with the SW margin of the Balingian oil Province which has been described by Swinburn (1993) as the West Balingian Line. The Balingian Shear zone is characterised by intensely folded turbidites belonging to the upper part of the Belaga Formation. Cleavage, quartz-filled jointing and ptygmatic folds, boudinage and small-scale thrusting are common within a belt about 5 km wide. Structural measurements obtained from the Balingian exposures indicate a general N -S compressional direction but more data are needed from a wider area before either the structural interpretation or lateral extent and direction of the shear zone, including whether there is any horizontal component, can be established satisfactorily. The timing of the deformation cannot be verified except that it is post upper Eocene. -

Anti-Inflammatory Potential of Hexane Extract of Mud Lobster (Thalassina Anomala) in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages
Anti-Inflammatory Potential of Hexane Extract of Mud Lobster (Thalassina anomala) in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages Authors: Nur Nadiah Zakaria, Masnindah Malahubban, Sharida Fakurazi, Wong Sie Chuong and Amy Halimah Rajaee* *Corresponding author: [email protected] DOI: https://doi.org/10.21315/tlsr2021.32.1.9 Highlights • All samples collected from Kuala Tatau, Bintulu; Kuala Balingian, Mukah and Sarikei were identified as Thalassina anomala. • The hexane extract of T. anomala exhibits anti-inflammatory potential in LPS-stimulated RAW 264.7 macrophages. • The non-polar compounds detected in the hexane extract of T. anomala by GC-MS analysis revealed 19 putative metabolites which may contribute to the anti-inflammatory activities. TLSR, 32(1), 2021 © Penerbit Universiti Sains Malaysia, 2021 Tropical Life Sciences Research, 32(1), 145–162, 2021 Anti-Inflammatory Potential of Hexane Extract of Mud Lobster (Thalassina anomala) in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages 1Nur Nadiah Zakaria, 1Masnindah Malahubban, 3Sharida Fakurazi, 2Wong Sie Chuong and 1,4Amy Halimah Rajaee* 1Department of Animal Science and Fishery, Faculty of Agricultural Science and Forestry, Universiti Putra Malaysia Bintulu Sarawak Campus, 97000 Bintulu, Sarawak, Malaysia 2Department of Science and Technology, Faculty of Humanities, Management and Science, Universiti Putra Malaysia Bintulu Sarawak Campus, 97000 Bintulu, Sarawak, Malaysia 3Department of Human Anatomy, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia 4Halal Products Research Institute, Universiti Putra Malaysia, Putra Infoport, 43400 UPM Serdang, Selangor, Malaysia Publication date: 31 March 2021 To cite this article: Nur Nadiah Zakaria, Masnindah Malahubban, Sharida Fakurazi, Wong Sie Chuong and Amy Halimah Rajaee. -

Strengthening Rural Economy Through Regional Development Planning Approach in Sarawak
International Journal of Academic Research in Business and Social Sciences Vol. 8 , No. 13, Special Issue: Community Development & Social Mobility, 2018, E-ISSN: 2222-6990 © 2018 HRMARS Strengthening Rural Economy through Regional Development Planning Approach in Sarawak Daniel U.E., Novel Lyndon, Suhana S., Sarmila M.S. & Zaimah, R. To Link this Article: http://dx.doi.org/10.6007/IJARBSS/v8-i13/4816 DOI: 10.6007/IJARBSS/v8-i13/4816 Received: 19 Sept 2018, Revised: 13 Oct 2018, Accepted: 02 Nov 2018 Published Online: 12 Nov 2018 In-Text Citation: (Daniel, Lyndon, Suhana, Sarmila, Zaimah 2018) To Cite this Article: Daniel, U.E., Lyndon, N., Suhana, S., Sarmila, M.S. & Zaimah, R. (2018). Strengthening Rural Economy through Regional Development Planning Approach in Sarawak. International Journal of Academic Research in Business and Social Sciences, 8(13 Special Issue: Community Development & Social Mobility), 122–129. Copyright: © 2018 The Author(s) Published by Human Resource Management Academic Research Society (www.hrmars.com) This article is published under the Creative Commons Attribution (CC BY 4.0) license. Anyone may reproduce, distribute, translate and create derivative works of this article (for both commercial and non-commercial purposes), subject to full attribution to the original publication and authors. The full terms of this license may be seen at: http://creativecommons.org/licences/by/4.0/legalcode Vol. 8, No. 13 – Special Issue: Community Development & Social Mobility, 2018, Pg. 122 - 129 http://hrmars.com/index.php/pages/detail/IJARBSS JOURNAL HOMEPAGE Full Terms & Conditions of access and use can be found at http://hrmars.com/index.php/pages/detail/publication-ethics 122 International Journal of Academic Research in Business and Social Sciences Vol. -

The Biology and Fisheries of the Slipper Lobster
The Biology and Fisheries of the Slipper Lobster Kari L. Lavalli College of General Studies Boston University Bo~ton, Massachusetts, U.S.A. Ehud Spanier The Leon Recanati Institute for Maritime Studies and Department of Maritime Civilizations University of Haifa Haifa, Israel 0 ~y~~F~~~~~oup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Cover image courtesy of Megan Elizabeth Stover of the College of General Studies, Boston University, Boston, Massachusetts. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2007 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number-10: 0-8493-3398-9 (Hardcover) International Standard Book Number-13: 978-0-8493-3398-9 (Hardcover) This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. No part ofthis book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any informa tion storage or retrieval system, without written permission from the publishers. -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest.