Calumma Boettgeri) with a Newly Defined Set of Morphometric and Meristic Measurements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
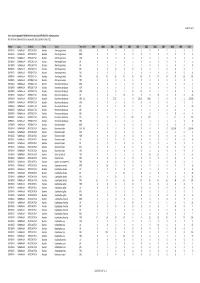
Gross Trade in Appendix II FAUNA (Direct Trade Only), 1999-2010 (For
AC25 Inf. 5 (1) Gross trade in Appendix II FAUNA (direct trade only), 1999‐2010 (for selection process) N.B. Data from 2009 and 2010 are incomplete. Data extracted 1 April 2011 Phylum Class TaxOrder Family Taxon Term Unit 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Total CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BOD 0 00001000102 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BON 0 00080000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia HOR 0 00000110406 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia LIV 0 00060000006 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKI 1 11311000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKP 0 00000010001 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKU 2 052101000011 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia TRO 15 42 49 43 46 46 27 27 14 37 26 372 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Antilope cervicapra TRO 0 00000020002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae BOD 0 00100001002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOP 0 00200000002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOR 0 0010100120216 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae LIV 0 0 5 14 0 0 0 30 0 0 0 49 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA KIL 0 5 27.22 0 0 272.16 1000 00001304.38 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA 0 00000000101 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae -

'True Chameleon' from Madagascar: a New, Distinctly Colored
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Zoosystematics and Evolution Jahr/Year: 2018 Band/Volume: 94 Autor(en)/Author(s): Prötzel David, Lambert Shea M., Andrianasolo Ginah Tsiorisoa, Hutter Carl R., Cobb Kerry A., Scherz Mark D., Glaw Frank Artikel/Article: The smallest ‘true chameleon’ from Madagascar: a new, distinctly colored species of the Calumma boettgeri complex (Squamata, Chamaeleonidae) 409-423 Creative Commons Attribution 4.0 licence (CC-BY); original download https://pensoft.net/journals Zoosyst. Evol. 94 (2) 2018, 409–423 | DOI 10.3897/zse.94.27305 The smallest ‘true chameleon’ from Madagascar: a new, distinctly colored species of the Calumma boettgeri complex (Squamata, Chamaeleonidae) David Prötzel1, Shea M. Lambert2, Ginah Tsiorisoa Andrianasolo3, Carl R. Hutter4, Kerry A. Cobb5, Mark D. Scherz1,6, Frank Glaw1 1 Zoologische Staatssammlung München (ZSM-SNSB), Münchhausenstr. 21, 81247 München, Germany 2 Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA 3 Mention Zoologie et Biodiversité Animale, Université d’Antananarivo, BP 906, Antananarivo 101, Madagascar 4 Biodiversity Institute and Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, KS 66045–7561, USA 5 Department of Biological Sciences, Auburn University, Auburn, AL 36849, USA 6 Zoologisches Institut, Technische Universität Braunschweig, Mendelssohnstr. 4, 38106 Braunschweig, Germany http://zoobank.org/2433A9DD-8AC1-4139-A639-E24053D5C33F Corresponding author: David Prötzel ([email protected]) Abstract Received 8 June 2018 On a recent expedition to eastern Madagascar, we discovered a distinct new species of the Accepted 10 August 2018 genus Calumma that we describe here using an integrative approach combining morphol- Published 19 October 2018 ogy, coloration, osteology and molecular genetics. -

Calumma Vohibola, a New Chameleon Species (Squamata: Chamaeleonidae) from the Littoral Forests of Eastern Madagascar Philip-Sebastian Gehring a , Fanomezana M
This article was downloaded by: [Sebastian Gehring] On: 26 October 2011, At: 23:51 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK African Journal of Herpetology Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/ther20 Calumma vohibola, a new chameleon species (Squamata: Chamaeleonidae) from the littoral forests of eastern Madagascar Philip-Sebastian Gehring a , Fanomezana M. Ratsoavina a b c , Miguel Vences a & Frank Glaw d a Division of Evolutionary Biology, Zoological Institute, Technical University of Braunschweig, Mendelssohnstr. 4, 38106, Braunschweig, Germany b Département de Biologie Animale, Université d'Antananarivo, BP 906, Antananarivo, 101, Madagascar c Grewcock Center for Conservation Research, Omaha's Henry Doorly Zoo, 3701 South 10th Street, Omaha, NE, 68107-2200, USA d Zoologische Staatssammlung München, Münchhausenstr. 21, 81247, München, Germany Available online: 26 Oct 2011 To cite this article: Philip-Sebastian Gehring, Fanomezana M. Ratsoavina, Miguel Vences & Frank Glaw (2011): Calumma vohibola, a new chameleon species (Squamata: Chamaeleonidae) from the littoral forests of eastern Madagascar, African Journal of Herpetology, 60:2, 130-154 To link to this article: http://dx.doi.org/10.1080/21564574.2011.628412 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.tandfonline.com/page/terms-and- conditions This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. -

No Longer Single! Description of Female Calumma Vatosoa (Squamata, Chamaeleonidae) Including a Review of the Species and Its Systematic Position
Zoosyst. Evol. 92 (1) 2016, 13–21 | DOI 10.3897/zse.92.6464 museum für naturkunde No longer single! Description of female Calumma vatosoa (Squamata, Chamaeleonidae) including a review of the species and its systematic position David Prötzel1, Bernhard Ruthensteiner1, Frank Glaw1 1 Zoologische Staatssammlung München (ZSM-SNSB), Münchhausenstr. 21, 81247 München, Germany http://zoobank.org/CFD64DFB-D085-4D1A-9AA9-1916DB6B4043 Corresponding author: David Prötzel ([email protected]) Abstract Received 3 September 2015 Calumma vatosoa is a Malagasy chameleon species that has until now been known only Accepted 26 November 2015 from the male holotype and a photograph of an additional male specimen. In this paper Published 8 January 2016 we describe females of the chameleon Calumma vatosoa for the first time, as well as the skull osteology of this species. The analysed females were collected many years before Academic editor: the description of C. vatosoa, and were originally described as female C. linotum. Ac- Johannes Penner cording to external morphology, osteology, and distribution these specimens are assigned to C. vatosoa. Furthermore we discuss the species group assignment of C. vatosoa and transfer it from the C. furcifer group to the C. nasutum group. A comparison of the exter- Key Words nal morphology of species of both groups revealed that C. vatosoa has a relatively shorter distance from the anterior margin of the orbit to the snout tip, more heterogeneous scala- Madagascar tion at the lower arm, a significantly lower number of supralabial and infralabial scales, chameleon and a relatively longer tail than the members of the C. furcifer group. -

CAMELEON Calumma Nasutum (DUMERIL & BIBRON, 1836) DE LA FORET DE MOYENNE ET DE BASSE ALTITUDE DE L’EST DE MADAGASCAR
UNIVERSITE D’ANTANANARIVO FACULTE DES SCIENCES DEPARTEMENT DE BIOLOGIE ANIMALE Latimeria chalumnae MEMOIRE POUR L’OBTENTION DU Diplôme d’Etudes Approfondies (D.E.A.) Formation Doctorale : Sciences de la Vie Option : Biologie, Ecologie et Conservation Animales ESSAI DE CLARIFICATION DE LA TAXONOMIE DU PETIT CAMELEON Calumma nasutum (DUMERIL & BIBRON, 1836) DE LA FORET DE MOYENNE ET DE BASSE ALTITUDE DE L’EST DE MADAGASCAR Présenté par : Mademoiselle Marie Paule RAZAFIMAHATRATRA Devant les membres du Jury composé de : Président : Madame Noromalala RASOAMAMPIONONA RAMINOSOA Professeur d’ESR Rapporteur : Monsieur Daniel RAKOTONDRAVONY Maître de Conférences Examinateurs : Monsieur Achille P. RASELIMANANA Professeur d’ESR Monsieur Hery A. RAKOTONDRAVONY Maître de Conférences Soutenu publiquement le 30 avril 2015 UNIVERSITE D’ANTANANARIVO FACULTE DES SCIENCES DEPARTEMENT DE BIOLOGIE ANIMALE Latimeria chalumnae MEMOIRE POUR L’OBTENTION DU Diplôme d’Etudes Approfondies (D.E.A.) Formation Doctorale : Sciences de la Vie Option : Biologie, Ecologie et Conservation Animales ESSAI DE CLARIFICATION DE LA TAXONOMIE DU PETIT CAMELEON Calumma nasutum (DUMERIL & BIBRON, 1836) DE LA FORET DE MOYENNE ET DE BASSE ALTITUDE DE L’EST DE MADAGASCAR Présenté par : Mademoiselle Marie Paule RAZAFIMAHATRATRA Devant les membres du Jury composé de : Président : Madame Noromalala RASOAMAMPIONONA RAMINOSOA Professeur d’ESR Rapporteur : Monsieur Daniel RAKOTONDRAVONY Maître de Conférences Examinateurs : Monsieur Achille P. RASELIMANANA Professeur d’ESR Monsieur Hery A. -

Habitat Use and Abundance of a Low-Altitude Chameleon Assemblage in Eastern Madagascar
HERPETOLOGICAL JOURNAL 17: 247–254, 2007 Habitat use and abundance of a low-altitude chameleon assemblage in eastern Madagascar Jeanneney Rabearivony1, Lee D. Brady2, Richard K.B. Jenkins3,4 & Olga R. Ravoahangimalala1 1Département de Biologie Animale, Université d’Antananarivo, Madagascar 2Durrell Institute of Conservation and Ecology, University of Kent, Canterbury, UK 3School of Biological Sciences, University of Aberdeen, UK 4Madagasikara Voakajy, Antananarivo, Madagascar We studied the density and abundance of chameleons in a lowland Malagasy rainforest during the austral summer and winter. Nocturnal searches for chameleons were conducted along transects within relatively intact forest and vegetation on abandoned agricultural land adjacent to the forest. Four chameleon species were encountered during the study, Brookesia superciliaris, Calumma parsonii parsonii, Calumma nasutum and Furcifer pardalis. Brookesia superciliaris was most common inside relatively intact forest and the few individuals located in the regenerating forest on abandoned agricultural land were found in tiny, isolated patches of degraded rainforest next to rivers. Calumma p. parsonii was only encountered on three occasions in relatively intact forest and was a rare member of the community. The abundance of C. nasutum was highest in relatively intact forest but this species also occurred in vegetation on abandoned agricultural land. Furcifer pardalis was only found on the abandoned agricultural land, where it was observed laying eggs in sandy soil in August. The abundance of all species in habitats alongside rivers was higher in January than July–August, with the exception of C. p. parsonii, which was not detected during the former period. Additional investigations into habitat preference of chameleons and surveys in other forests in region are needed to establish whether the low abundance of C. -

Population Assessments of Chameleons from Two Montane Sites in Madagascar
Herpetological Conservation and Biology 5(1):23-31. Submitted: 9 September 2009; Accepted: 23 November 2009. POPULATION ASSESSMENTS OF CHAMELEONS FROM TWO MONTANE SITES IN MADAGASCAR 1 1 J. CHRISTIAN RANDRIANANTOANDRO , RAPHALI R. ANDRIANTSIMANARILAFY , 2 3 HERIZO RAKOTOVOLOLONALIMANANA , ELISOA F. HANTALALAINA , 3 3 2 DANIEL RAKOTONDRAVONY , OLGA R. RAMILIJAONA , JONAH RATSIMBAZAFY , 3 1,4,5 GERMAIN F. RAZAFINDRAKOTO , AND RICHARD K. B. JENKINS 1Madagasikara Voakajy, B.P. 5181, Antananarivo (101), Madagascar, e-mail: [email protected] 2Ecole Supérieure des Sciences Agronomiques, Département des Eaux et Forêts, Université d’Antananarivo, Antananarivo (101), Madagascar 3Department of Animal Biology, University of Antananarivo, Antananarivo, Madagascar 4School of Biological Sciences, University of Aberdeen, Aberdeen, AB24 2TZ, United Kingdom 5Current address: Durrell Institute of Conservation and Ecology, School of Anthropology and Conservation, University of Kent, Canterbury, United Kingdom Abstract.—The highest peaks of Madagascar contain relatively little native forest vegetation but they have a remarkably high endemicity and diversity of reptiles and amphibians for their surface area. Many chameleons are associated with these highland habitats in Madagascar and we assessed the population status of five species at Itremo- Ambatofinandrahana and Ankaratra, including three with restricted ranges (Calumma hilleniusi, Furcifer campani and Furcifer minor). Calumma species occurred at relatively high densities and were restricted to humid forest with the occasional occurrence of C. hilleniusi in savanna. Furcifer minor and Furcifer lateralis were common in both tapia (Uapaca spp.) forest and agricultural land while F. campani was restricted to savanna and heathland. Calumma hilleniusi is of conservation concern because of its restricted range and dwindling habitat. Furcifer campani and F. -

Amphibian and Reptile Records from Lowland Rainforests in Eastern Madagascar
SALAMANDRA 46(4) 214–234 20 NovemberPhilip-Sebastian 2010 ISSN Gehring 0036–3375 et al. Filling the gaps – amphibian and reptile records from lowland rainforests in eastern Madagascar Philip-Sebastian Gehring1, Fanomezana M. Ratsoavina1,2,3 & Miguel Vences1 1) Technical University of Braunschweig, Zoological Institute, Spielmannstr. 8, 38106 Braunschweig, Germany 2) Département de Biologie Animale, Université d’Antananarivo, BP 906. Antananarivo, 101, Madagascar 3) Grewcock Center for Conservation Research, Omaha´s Henry Doorly Zoo, 3701 South 10th Street, Omaha, NE 68107-2200, U.S.A. Corresponding author: Philip-Sebastian Gehring, e-mail: [email protected] Manuscript received: 27 May 2010 Abstract. We report on the results of a survey of amphibians and reptiles at several primary and secondary lowland habi- tats along Madagascar’s east coast. The survey yielded a total of 106 species (61 amphibians and 45 reptiles). Comparisons of mitochondrial DNA sequences of selected amphibian and reptile species confirmed their identification and in some cases allowed to assign them to particular intraspecific genetic lineages. The highest species diversity was found in the pri- mary lowland rainforests of Ambodiriana and Sahafina. The littoral forests of Tampolo and Vohibola held overall a higher species diversity than the anthropogenic secondary forest formations of Vatomandry and Mahanoro. Structural differ- ences between lowland forests and littoral forests seem to cause a difference in species composition, especially relevant for the amphibian species assemblages. Besides a number of undescribed species, the most remarkable records were those of Mantidactylus majori, Uroplatus lineatus and Blaesodactylus antongilensis in the Sahafina forest at Madagascar’s central east coast, which constitute significant range extensions for these species. -

Spatialisation Des Donnees Ecologiques De La Vegetation Pour La Conservation Des Unites Paysageres De La Peninsule D’Ampasindava
Remerciements - UNIVERSITE D’ANTANANARIVO DOMAINE DES SCIENCES ET TECHNOLOGIES ECOLE DOCTORALE DES SCIENCES DE LA VIE ET DE L’ENVIRONNEMENT THESE POUR L’OBTENTION DU DIPLOME DE DOCTORAT EN SCIENCES DE LA VIE ET DE L’ENVIRONNEMENT SPECIALITE : SCIENCES DU VEGETAL SPATIALISATION DES DONNEES ECOLOGIQUES DE LA VEGETATION POUR LA CONSERVATION DES UNITES PAYSAGERES DE LA PENINSULE D’AMPASINDAVA Présenté par Jacquis A. TAHINARIVONY Soutenue publiquement le 7 novembre 2016 devant le jury composé de Président : Professeur RAKOUTH Bakolimalala Directeur de thèse : Professeur RAKOTOARIMANANA Vonjison Co-Directeur : Docteur GAUTIER Laurent Rapporteur interne : Professeur FARAMALALA Miadana Harisoa Rapporteur externe : Professeur RAKOTONDRAOMPIANA Solofo Examinateur : Professeur RAZANAKA Samuel Invité : Docteur ROGER Edmond i Remerciements REMERCIEMENTS Cette thèse est le fruit de plusieurs années de recherches, d’une longue traversée semée de découvertes, de difficultés et d’expériences inestimables. Pendant ces années, de nombreuses initiatives et efforts se sont associés pour contribuer au bon déroulement de ce travail par des personnes et institutions. Je ne saurais les oublier et j’aimerais témoigner ma reconnaissance et mes remerciements envers eux. - Professeur Bakolimalala RAKOUTH, Enseignant chercheur au Département de Biologie et Ecologie Végétales qui m’a fait l’honneur de présider cette thèse. - Professeur Vonjison RAKOTOARIMANANA, Enseignant chercheur au Département de Biologie et Ecologie Végétales, de m’avoir fait le grand honneur de diriger cette thèse et d’avoir prodigué de judicieux conseils. - Docteur Laurent GAUTIER, des Conservatoires et Jardin Botaniques de Genève, qui m’a encadré depuis la période de collecte des données sur le terrain jusqu’à la rédaction du travail. Ses partages de connaissances et expériences en flore de Madagascar et à la compréhension des différentes approches et principes d’analyse des données écologiques m’ont été précieux. -

Review of Calumma and Furcifer Species from Madagascar Species Subject to Increased Quotas in 2014 Following Removal of Long-Standing CITES and EU Suspensions
UNEP-WCMC technical report Review of Calumma and Furcifer species from Madagascar Species subject to increased quotas in 2014 following removal of long-standing CITES and EU suspensions (Version edited for public release) Review of Calumma and Furcifer species from Madagascar. Species subject to increased quotas in 2014 following removal of long- 2 standing CITES and EU suspensions Prepared for The European Commission, Directorate General Environment, Directorate E - Global & Regional Challenges, LIFE ENV.E.2. – Global Sustainability, Trade & Multilateral Agreements, Brussels, Belgium Prepared June 2015 Copyright European Commission 2015 Citation UNEP-WCMC. 2015. Review of Calumma and Furcifer species from Madagascar. Species subject to increased quotas in 2014 following removal of long-standing CITES and EU suspensions. UNEP- WCMC, Cambridge. The UNEP World Conservation Monitoring Centre (UNEP-WCMC) is the specialist biodiversity assessment of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organization. The Centre has been in operation for over 30 years, combining scientific research with policy advice and the development of decision tools. We are able to provide objective, scientifically rigorous products and services to help decision- makers recognize the value of biodiversity and apply this knowledge to all that they do. To do this, we collate and verify data on biodiversity and ecosystem services that we analyze and interpret in comprehensive assessments, making the results available in appropriate forms for national and international level decision-makers and businesses. To ensure that our work is both sustainable and equitable we seek to build the capacity of partners where needed, so that they can provide the same services at national and regional scales. -

Systematic Revision of the Malagasy Chameleons Calumma Boettgeri and C
Zootaxa 4048 (2): 211–231 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.4048.2.4 http://zoobank.org/urn:lsid:zoobank.org:pub:2CAB0746-175E-4FE1-B0D0-23DFF395A559 Systematic revision of the Malagasy chameleons Calumma boettgeri and C. linotum (Squamata: Chamaeleonidae) DAVID PRÖTZEL1,2, BERNHARD RUTHENSTEINER1, MARK D. SCHERZ1 & FRANK GLAW1 1Zoologische Staatssammlung München (ZSM-SNSB), Münchhausenstr. 21, 81247 München, Germany 2Corresponding author. E-mail: [email protected] Abstract We revise the taxonomic status of two species of Madagascan chameleons in light of a recent molecular phylogenetic study on the Calumma nasutum group. The investigation of morphological and osteological characters led to a clear de- lineation between two species within the C. boettgeri complex, C. boettgeri and C. linotum. Calumma linotum has been considered either a synonym of C. boettgeri or a dubious, poorly defined taxon. So far it has only been known from the male holotype with the imprecise locality ‘Madagascar’. Based on pholidosis, morphological measurements and charac- ters of the skull that were analyzed using micro-X-ray computed tomography (micro-CT) scans, we ascribe the population of chameleons from Montagne d’Ambre, formerly assigned to C. boettgeri, to C. linotum. Calumma linotum differs from C. boettgeri in the larger size of tubercle scales on the extremities and rostral appendage, the larger diameter of the ex- tremities relative to the body size, the presence of a parietal crest as well as the form of the nasal bones and the anterior tip of the frontal. -
Furcifer and Calumma Were Assessed by a Consultant and Each Taxon Was Attributed to One of Four Categories
CLP Project ID Code F210308 Project Title A Conservation Framework for Furcifer Chameleons in Madagascar Host country Madagascar Site location - Ankaratra massif (Central highland) - Bongolava Forest corridor (North-Western) - Belalanda and Sakabera (South-Western) - Angavokely Forestry Station (Central highland) - Tsitongambarika Forest (South-Eastern) Dates in the - October 2008 (in Belalanda and Sakabera) field - November-December 2008 (in Ankaratra massif) - March-April 2009 (in Belalanda and Sakabera) - October 2009 (in Ankaratra massif) - November-December 2009 (in Belalanda and Sakabera) - November 2009 (in Tsitongambarika Forest) - January 2010 (in Ankaratra Massif) - March 2010 (in Bongolava forest) Institutions - Conservation International Madagascar) partners - World Wildlife Fund Madagascar West Indian Ocean region (WWF MWIO) - International Union for Conservation of Nature (IUCN) - Department of Animal Biology Antananarivo University Madagascar (DBA-UA) - Département des Sciences Biologiques Tulear University (DSB- TU) - Durrell Institute of Conservation and Ecology (DICE) - Direction de la Valorisation des Ressources Naturelles of Ministry of Environment and Forests Madagascar (DVRN/MEF) - Direction de la Conservation de la Biodiversité et du Système des Aires Protégées of Ministry of Environment and Forests Madagascar (DCBSAP/MEF) Project Aim To conserve the endemic Furcifer chameleons of Madagascar Authors - Christian Joseph RANDRIANANTOANDRO - Raphali Rodlis ANDRIANTSIMANARILAFY - Roma RANDRIANAVELONA Address B.P. 5181,