Habitat Use and Abundance of a Low-Altitude Chameleon Assemblage in Eastern Madagascar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extreme Miniaturization of a New Amniote Vertebrate and Insights Into the Evolution of Genital Size in Chameleons
www.nature.com/scientificreports OPEN Extreme miniaturization of a new amniote vertebrate and insights into the evolution of genital size in chameleons Frank Glaw1*, Jörn Köhler2, Oliver Hawlitschek3, Fanomezana M. Ratsoavina4, Andolalao Rakotoarison4, Mark D. Scherz5 & Miguel Vences6 Evolutionary reduction of adult body size (miniaturization) has profound consequences for organismal biology and is an important subject of evolutionary research. Based on two individuals we describe a new, extremely miniaturized chameleon, which may be the world’s smallest reptile species. The male holotype of Brookesia nana sp. nov. has a snout–vent length of 13.5 mm (total length 21.6 mm) and has large, apparently fully developed hemipenes, making it apparently the smallest mature male amniote ever recorded. The female paratype measures 19.2 mm snout–vent length (total length 28.9 mm) and a micro-CT scan revealed developing eggs in the body cavity, likewise indicating sexual maturity. The new chameleon is only known from a degraded montane rainforest in northern Madagascar and might be threatened by extinction. Molecular phylogenetic analyses place it as sister to B. karchei, the largest species in the clade of miniaturized Brookesia species, for which we resurrect Evoluticauda Angel, 1942 as subgenus name. The genetic divergence of B. nana sp. nov. is rather strong (9.9‒14.9% to all other Evoluticauda species in the 16S rRNA gene). A comparative study of genital length in Malagasy chameleons revealed a tendency for the smallest chameleons to have the relatively largest hemipenes, which might be a consequence of a reversed sexual size dimorphism with males substantially smaller than females in the smallest species. -

Zootaxa, Integrative Taxonomy of Malagasy Treefrogs
Zootaxa 2383: 1–82 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) ZOOTAXA 2383 Integrative taxonomy of Malagasy treefrogs: combination of molecular genetics, bioacoustics and comparative morphology reveals twelve additional species of Boophis FRANK GLAW1, 5, JÖRN KÖHLER2, IGNACIO DE LA RIVA3, DAVID R. VIEITES3 & MIGUEL VENCES4 1Zoologische Staatssammlung München, Münchhausenstr. 21, 81247 München, Germany 2Department of Natural History, Hessisches Landesmuseum Darmstadt, Friedensplatz 1, 64283 Darmstadt, Germany 3Museo Nacional de Ciencias Naturales-Consejo Superior de Investigaciones Científicas (CSIC), C/ José Gutiérrez Abascal 2, 28006 Madrid, Spain 4Zoological Institute, Technical University of Braunschweig, Spielmannstr. 8, 38106 Braunschweig, Germany 5Corresponding author. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by S. Castroviejo: 8 Dec. 2009; published: 26 Feb. 2010 Frank Glaw, Jörn Köhler, Ignacio De la Riva, David R. Vieites & Miguel Vences Integrative taxonomy of Malagasy treefrogs: combination of molecular genetics, bioacoustics and com- parative morphology reveals twelve additional species of Boophis (Zootaxa 2383) 82 pp.; 30 cm. 26 February 2010 ISBN 978-1-86977-485-1 (paperback) ISBN 978-1-86977-486-8 (Online edition) FIRST PUBLISHED IN 2010 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2010 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. -

Care Sheet for the Panther Chameleon Furcifer Pardalis By
Care Sheet for the Panther Chameleon Furcifer pardalis By Petr Necas & Bill Strand Legend Sub-legend Description Taxon Furcifer pardalis Panther Chameleon (English) Common Names Sakorikita (Malagassy) Original name Chamaeleo pardalis Author Cuvier, 1829 Original description Règne, animal, 2nd ed., 2: 60 Type locality Ile de France (= Mauritius, erroneous), restricted to Madagascar Typus HNP 6520 A formally monotypic species with no recognized subspecies, however recent studies reveal many (4 big, up to 11) entities within this species, defined geographically, that show different level of relativeness, some so distant from each other to be possibly con- sidered a separate species and/or subspecies. Taxonomy Historically, many synonyms were introduced, such as Chamaeleo ater, niger, guen- theri, longicauda, axillaris, krempfi. The term “locale” is used in captive management only; it has no taxonomic relevance and refers to the distinct subpopulations named usually after a village within its (often not isolated and well defined) range, differing from each other through unique color- Taxonomy ation and patterns, mainly males. The distinguished “locales” are as follows: Ambanja, Ambilobe, Ampitabe, Androngombe, Ankaramy, Ankarana (E and W), Andapa, Anki- fy, Antalaha, Antsiranana (Diego Suarez), Beramanja, Cap Est, Djangoa, Fenoarivo, Mahavelona, Mangaoka, Manambato, Mananara, Maroantsetra, Marojejy, Nosy Be, Nosy Boraha, Nosy Faly, Nosy Mangabe, Nosy Mitsio, Sambava, Sambirano, Soanier- ana Ivongo, Toamasina (Tamatave), Vohimana. Captive projects include often delib- erate crossbreeding of “locales” that lead to genetically unidentifiable animals and should be omitted. Member of the genus Furcifer. 2 Legend Sub-legend Description Distributed along NE, N, NW and E coast of Madagascar, south reaching the vicinity of Tamatave, including many offshore islands (e.g. -

MADAGASCAR: the Wonders of the “8Th Continent” a Tropical Birding Custom Trip
MADAGASCAR: The Wonders of the “8th Continent” A Tropical Birding Custom Trip October 20—November 6, 2016 Guide: Ken Behrens All photos taken during this trip by Ken Behrens Annotated bird list by Jerry Connolly TOUR SUMMARY Madagascar has long been a core destination for Tropical Birding, and with the opening of a satellite office in the country several years ago, we further solidified our expertise in the “Eighth Continent.” This custom trip followed an itinerary similar to that of our main set-departure tour. Although this trip had a definite bird bias, it was really a general natural history tour. We took our time in observing and photographing whatever we could find, from lemurs to chameleons to bizarre invertebrates. Madagascar is rich in wonderful birds, and we enjoyed these to the fullest. But its mammals, reptiles, amphibians, and insects are just as wondrous and accessible, and a trip that ignored them would be sorely missing out. We also took time to enjoy the cultural riches of Madagascar, the small villages full of smiling children, the zebu carts which seem straight out of the Middle Ages, and the ingeniously engineered rice paddies. If you want to come to Madagascar and see it all… come with Tropical Birding! Madagascar is well known to pose some logistical challenges, especially in the form of the national airline Air Madagascar, but we enjoyed perfectly smooth sailing on this tour. We stayed in the most comfortable hotels available at each stop on the itinerary, including some that have just recently opened, and savored some remarkably good food, which many people rank as the best Madagascar Custom Tour October 20-November 6, 2016 they have ever had on any birding tour. -

Blumgart Et Al 2017- Herpetological Survey Nosy Komba
Journal of Natural History ISSN: 0022-2933 (Print) 1464-5262 (Online) Journal homepage: http://www.tandfonline.com/loi/tnah20 Herpetological diversity across intact and modified habitats of Nosy Komba Island, Madagascar Dan Blumgart, Julia Dolhem & Christopher J. Raxworthy To cite this article: Dan Blumgart, Julia Dolhem & Christopher J. Raxworthy (2017): Herpetological diversity across intact and modified habitats of Nosy Komba Island, Madagascar, Journal of Natural History, DOI: 10.1080/00222933.2017.1287312 To link to this article: http://dx.doi.org/10.1080/00222933.2017.1287312 Published online: 28 Feb 2017. Submit your article to this journal Article views: 23 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tnah20 Download by: [BBSRC] Date: 21 March 2017, At: 02:56 JOURNAL OF NATURAL HISTORY, 2017 http://dx.doi.org/10.1080/00222933.2017.1287312 Herpetological diversity across intact and modified habitats of Nosy Komba Island, Madagascar Dan Blumgart a, Julia Dolhema and Christopher J. Raxworthyb aMadagascar Research and Conservation Institute, BP 270, Hellville, Nosy Be, Madagascar; bDivision of Vertebrate Zoology, American, Museum of Natural History, New York, NY, USA ABSTRACT ARTICLE HISTORY A six month herpetological survey was undertaken between March Received 16 August 2016 and September 2015 on Nosy Komba, an island off of the north- Accepted 17 January 2017 west coast of mainland Madagascar which has undergone con- KEYWORDS fi siderable anthropogenic modi cation. A total of 14 species were Herpetofauna; conservation; found that have not been previously recorded on Nosy Komba, Madagascar; Nosy Komba; bringing the total island diversity to 52 (41 reptiles and 11 frogs). -

Parson's Chameleon
L’ARCHE LES ÉCODOCS PHOTOGRAPHIQUE endangered species… ACTIONS FOR BIODIVERSITY PARSON’S CHAMELEON CHARACTERISTICS Kingdom Animal he chameleon is the unchallenged Phylum Chordata master of camouflage, and Parson’s Class Reptilia chameleon is by far the largest spe- Order Squamata cimen in Madagascar and on the Family Chamæleonidæ Tplanet! The endemic species lives only in the tro- Genus Calumma pical forests in the east of the Red Island. It can Latin name Calumma parsonii be easily recognised by its size. The adult has two Weight up to 1 kg outgrowths at the end of its muzzle, and the back Size 90 cm of its head extends in the shape of a helmet. Its Incubation period 21 months Number of eggs 20-25 limbs are stronger and more robust than those Interval between reproduction 2 years of all its fellows. It uses its small horns when it Diet Insectivore fights other males for a female. Longevity 4 years CITES Annexe II PAGE 1/3 LES ÉCODOCS PARSON’S endangered species… CHAMELEON Parson’s chameleon (Calumma parsonii) on the look-out. During a fight, the adversaries change from tur- that gives it a perfect estimate of its distance. Like quoise blue to threatening red. The vanquished a sucker, it catches its prey with the muscled tip male darkens his colours and goes flat on its of its large tongue covered with sticky saliva. Its belly under the branch. Conversely, the victor puts on even more flamboyant colours. The female couples only once every two years. Oviparous, she digs the soil in which to lay her eggs. -

Furcifer Cephalolepis Günther, 1880
AC22 Doc. 10.2 Annex 7 Furcifer cephalolepis Günther, 1880 FAMILY: Chamaeleonidae COMMON NAMES: Comoro Islands Chameleon (English); Caméléon des Comores (French) GLOBAL CONSERVATION STATUS: Not yet assessed by IUCN. SIGNIFICANT TRADE REVIEW FOR: Comoros Range State selected for review Range State Exports* Urgent, Comments (1994-2003) possible or least concern Comoros 7,150 Least Locally abundant. No trade recorded since 1993, when only 300 exported. concern No known monitoring or evidence of non-detriment findings. *Excluding re-exports SUMMARY Furcifer cephalolepis is a relatively small chameleon endemic to the island of Grand Comoro (Ngazidja) in the Comoros, where it occurs at altitudes of between 300 m and 650 m, and has an area of occupancy of between 300 km2 and 400 km2. It occurs in disturbed and secondary vegetation, including in towns, and can reportedly be locally abundant, although no quantitative measures of population size are available. Plausible estimates indicate that populations may be in the range of tens of thousands to hundreds of thousands. The species is exported as a live animal for the pet trade. Recorded exports from the Comoros began in 2000 and, between then and 2003, some 7,000 animals were recorded as exported, latterly almost all to the USA. Only 300 were recorded in trade in 2003, and none in 2004 (or, to date, in 2005), despite the fact that exports of other Comorean reptiles, which dropped to a low or zero level in 2003, began again in 2004. Captive breeding has taken place, in the USA at least. The species is not known to be covered by any national legislation. -

Does Selective Wood Exploitation Affect Amphibian Diversity? the Case of An’Ala, a Tropical Rainforest in Eastern Madagascar
Oryx Vol 38 No 4 October 2004 Does selective wood exploitation affect amphibian diversity? The case of An’Ala, a tropical rainforest in eastern Madagascar Denis Vallan, Franco Andreone, Vola H. Raherisoa and Rainer Dolch Abstract The diversity of amphibians before and rainforest habitat showed a non-significant 10.1% after low-level forest exploitation in An’Ala forest in decrease in abundance after logging. It appears therefore central-eastern Madagascar was compared over the that amphibians are relatively resilient to a low-level course of 4 years. Neither abundance nor diversity of of forest exploitation and their diversity is apparently not amphibians generally were significantly affected by affected, at least in the short-term. This and other studies low-level selective logging, although the abundance of have, however, shown that logging commonly results individual species differed. Mantelline anurans were in a shift in species composition, with species typical of the most sensitive, in contrast to the tree frogs of the pristine rainforests being replaced by species adapted to subfamily Boophinae (Mantellidae) and Cophylinae secondary habitats. (Microhylidae). The abundance of Mantellinae anurans decreased by 15.8% after logging, whereas Boophinae Keywords Amphibian, Boophinae, Cophylinae, and Microhylidae anurans increased by 12.1% and diversity, Mantellinae, rainforest, selective wood 3.7%, respectively. In general, species strongly tied to exploitation. Introduction deforestation upon natural animal communities is urgently needed. Tropical rainforests worldwide are cleared and exploited Despite the existence of 16 protected areas in the for many reasons, and trees are often felled selectively, eastern rainforests (ANGAP, 2001), the vast majority of especially if the aim is to remove the most valuable the remaining natural vegetation does not have legal timber rather than clear felling for pasture and/or crop protection. -

Correlates of Eye Colour and Pattern in Mantellid Frogs
SALAMANDRA 49(1) 7–17 30Correlates April 2013 of eyeISSN colour 0036–3375 and pattern in mantellid frogs Correlates of eye colour and pattern in mantellid frogs Felix Amat 1, Katharina C. Wollenberg 2,3 & Miguel Vences 4 1) Àrea d‘Herpetologia, Museu de Granollers-Ciències Naturals, Francesc Macià 51, 08400 Granollers, Catalonia, Spain 2) Department of Biology, School of Science, Engineering and Mathematics, Bethune-Cookman University, 640 Dr. Mary McLeod Bethune Blvd., Daytona Beach, FL 32114, USA 3) Department of Biogeography, Trier University, Universitätsring 15, 54286 Trier, Germany 4) Zoological Institute, Division of Evolutionary Biology, Technical University of Braunschweig, Spielmannstr. 8, 38106 Braunschweig, Germany Corresponding author: Miguel Vences, e-mail: [email protected] Manuscript received: 18 March 2013 Abstract. With more than 250 species, the Mantellidae is the most species-rich family of frogs in Madagascar. These frogs are highly diversified in morphology, ecology and natural history. Based on a molecular phylogeny of 248 mantellids, we here examine the distribution of three characters reflecting the diversity of eye colouration and two characters of head colouration along the mantellid tree, and their correlation with the general ecology and habitat use of these frogs. We use Bayesian stochastic character mapping, character association tests and concentrated changes tests of correlated evolu- tion of these variables. We confirm previously formulated hypotheses of eye colour pattern being significantly correlated with ecology and habits, with three main character associations: many tree frogs of the genus Boophis have a bright col- oured iris, often with annular elements and a blue-coloured iris periphery (sclera); terrestrial leaf-litter dwellers have an iris horizontally divided into an upper light and lower dark part; and diurnal, terrestrial and aposematic Mantella frogs have a uniformly black iris. -

Madagascar: the Red Island
Andrea L. Baden & Rachel L. Jacobs Stony Brook University Taxonomic group Total species Endemic species % Endemism Plants 13,000 11,600 89.2 Mammals 155 144 92.9 Birds 310 181 58.4 Reptiles 384 367 95.6 Amphibians 230 229 99.6 Freshwater fish 164 97 59.1 *Recently extinct species: 45 (including birds, reptiles, and mammals) “The ecological state of being unique to a particular geographic location, such as a specific island…[Endemic species are] only found in that part of the world and nowhere else.” Taxonomic group Total species Endemic species % Endemism Plants 13,000 11,600 89.2 Mammals 155 144 92.9 Birds 310 181 58.4 Reptiles 384 367 95.6 Amphibians 230 229 99.6 Freshwater fish 164 97 59.1 *Recently extinct species: 45 (including birds, reptiles, and mammals) North & Central America . Phillippenes . California floristic province . Polynesia-Micronesia . Caribbean Islands . Southwest Australia . Madrean Pine Oak Woodlands . Sundaland . Mesoamerica . Wallaceae South America . Western Ghats & Sri Lanka . Atlantic Forest Europe & Central Asia . Cerrado . Caucasus . Chilean winter-Rainfall-Valdivian . Irano-Antalian forests . Mediterranean Basin . Tumbes-Choco-Magdalena . Mtns of Central Asia . Tropical Andes Africa Asia-Pacific . Cape Floristic region . E. Melanesian Islands . E. African coastal forests . Himalaya . Eastern afromontane . Indo-Burma . W. African Guinean forests . Japan . Horn of Africa . Mtns of SW China . Madagascar . New Caledonia . Maputaland-Pondoland-Albany . New Zealand . Succulent Karoo > 44% of the world’s plant species > 35% of the world’s terrestrial vertebrates Cover ~ 1.4% of the earth’s surface . was once 11%, but 88% of that has since been lost Madagascar contains 1 of 6 major radiations of primates . -
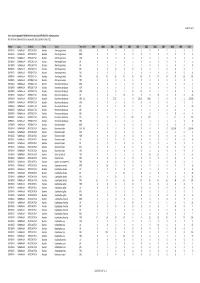
Gross Trade in Appendix II FAUNA (Direct Trade Only), 1999-2010 (For
AC25 Inf. 5 (1) Gross trade in Appendix II FAUNA (direct trade only), 1999‐2010 (for selection process) N.B. Data from 2009 and 2010 are incomplete. Data extracted 1 April 2011 Phylum Class TaxOrder Family Taxon Term Unit 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Total CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BOD 0 00001000102 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BON 0 00080000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia HOR 0 00000110406 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia LIV 0 00060000006 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKI 1 11311000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKP 0 00000010001 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKU 2 052101000011 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia TRO 15 42 49 43 46 46 27 27 14 37 26 372 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Antilope cervicapra TRO 0 00000020002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae BOD 0 00100001002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOP 0 00200000002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOR 0 0010100120216 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae LIV 0 0 5 14 0 0 0 30 0 0 0 49 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA KIL 0 5 27.22 0 0 272.16 1000 00001304.38 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA 0 00000000101 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae -

Description of a New Pygmy Chameleon (Chamaeleonidae: Brookesia) from Central Madagascar
Zootaxa 3490: 63–74 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2012 · Magnolia Press Article ISSN 1175-5334 (online edition) urn:lsid:zoobank.org:pub:FF22F75B-4A07-40D9-9609-1B8D269A921C Description of a new pygmy chameleon (Chamaeleonidae: Brookesia) from central Madagascar ANGELICA CROTTINI1,2,5, AURÉLIEN MIRALLES2, FRANK GLAW3, D. JAMES HARRIS1, ALEXANDRA LIMA1,4 & MIGUEL VENCES2 1CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Campus Agrário de Vairão, R. Padre Armando Quintas, 4485-661 Vairão, Portugal. E-mail: [email protected] 2Zoological Institute, Division of Evolutionary Biology, Technical University of Braunschweig, Mendelssohnstraße 4, 38106 Braunschweig, Germany 3Zoologische Staatssammlung München, Münchhausenstr. 21, 81247 München, Germany 4Departamento de Biologia, Faculdade de Ciências da Universidade do Porto, R. Campo Alegre s/n, 4169-007 Porto, Portugal 5Corresponding author Abstract We describe a new Brookesia species from a forest fragment located 13 km south of Ambalavao in the southern part of Madagascar's central high plateau. Brookesia brunoi sp. nov. is one of the few arid-adapted Brookesia species inhabiting deciduous forests on the western slope of the central high plateau of the island (around 950 m a.s.l.). So far the species has only been observed in the private Anja Reserve. The species belongs to the Brookesia decaryi group formed by arid-adapt- ed Brookesia species of western Madagascar: B. bonsi Ramanantsoa, B. perarmata (Angel), B. brygooi Raxworthy & Nussbaum and B. decaryi Angel. Brookesia brunoi differs from the other four species of the group by a genetic divergence of more than 17.6% in the mitochondrial ND2 gene, and by a combination of morphological characters: (1) nine pairs of laterovertebral pointed tubercles, (2) absence of enlarged pointed tubercles around the vent, (3) presence of poorly defined laterovertebral tubercles along the entire tail, (4) by the configuration of its cephalic crest, and (5) hemipenial morphology.