No Longer Single! Description of Female Calumma Vatosoa (Squamata, Chamaeleonidae) Including a Review of the Species and Its Systematic Position
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extreme Miniaturization of a New Amniote Vertebrate and Insights Into the Evolution of Genital Size in Chameleons
www.nature.com/scientificreports OPEN Extreme miniaturization of a new amniote vertebrate and insights into the evolution of genital size in chameleons Frank Glaw1*, Jörn Köhler2, Oliver Hawlitschek3, Fanomezana M. Ratsoavina4, Andolalao Rakotoarison4, Mark D. Scherz5 & Miguel Vences6 Evolutionary reduction of adult body size (miniaturization) has profound consequences for organismal biology and is an important subject of evolutionary research. Based on two individuals we describe a new, extremely miniaturized chameleon, which may be the world’s smallest reptile species. The male holotype of Brookesia nana sp. nov. has a snout–vent length of 13.5 mm (total length 21.6 mm) and has large, apparently fully developed hemipenes, making it apparently the smallest mature male amniote ever recorded. The female paratype measures 19.2 mm snout–vent length (total length 28.9 mm) and a micro-CT scan revealed developing eggs in the body cavity, likewise indicating sexual maturity. The new chameleon is only known from a degraded montane rainforest in northern Madagascar and might be threatened by extinction. Molecular phylogenetic analyses place it as sister to B. karchei, the largest species in the clade of miniaturized Brookesia species, for which we resurrect Evoluticauda Angel, 1942 as subgenus name. The genetic divergence of B. nana sp. nov. is rather strong (9.9‒14.9% to all other Evoluticauda species in the 16S rRNA gene). A comparative study of genital length in Malagasy chameleons revealed a tendency for the smallest chameleons to have the relatively largest hemipenes, which might be a consequence of a reversed sexual size dimorphism with males substantially smaller than females in the smallest species. -

Care Sheet for the Panther Chameleon Furcifer Pardalis By
Care Sheet for the Panther Chameleon Furcifer pardalis By Petr Necas & Bill Strand Legend Sub-legend Description Taxon Furcifer pardalis Panther Chameleon (English) Common Names Sakorikita (Malagassy) Original name Chamaeleo pardalis Author Cuvier, 1829 Original description Règne, animal, 2nd ed., 2: 60 Type locality Ile de France (= Mauritius, erroneous), restricted to Madagascar Typus HNP 6520 A formally monotypic species with no recognized subspecies, however recent studies reveal many (4 big, up to 11) entities within this species, defined geographically, that show different level of relativeness, some so distant from each other to be possibly con- sidered a separate species and/or subspecies. Taxonomy Historically, many synonyms were introduced, such as Chamaeleo ater, niger, guen- theri, longicauda, axillaris, krempfi. The term “locale” is used in captive management only; it has no taxonomic relevance and refers to the distinct subpopulations named usually after a village within its (often not isolated and well defined) range, differing from each other through unique color- Taxonomy ation and patterns, mainly males. The distinguished “locales” are as follows: Ambanja, Ambilobe, Ampitabe, Androngombe, Ankaramy, Ankarana (E and W), Andapa, Anki- fy, Antalaha, Antsiranana (Diego Suarez), Beramanja, Cap Est, Djangoa, Fenoarivo, Mahavelona, Mangaoka, Manambato, Mananara, Maroantsetra, Marojejy, Nosy Be, Nosy Boraha, Nosy Faly, Nosy Mangabe, Nosy Mitsio, Sambava, Sambirano, Soanier- ana Ivongo, Toamasina (Tamatave), Vohimana. Captive projects include often delib- erate crossbreeding of “locales” that lead to genetically unidentifiable animals and should be omitted. Member of the genus Furcifer. 2 Legend Sub-legend Description Distributed along NE, N, NW and E coast of Madagascar, south reaching the vicinity of Tamatave, including many offshore islands (e.g. -

MADAGASCAR: the Wonders of the “8Th Continent” a Tropical Birding Custom Trip
MADAGASCAR: The Wonders of the “8th Continent” A Tropical Birding Custom Trip October 20—November 6, 2016 Guide: Ken Behrens All photos taken during this trip by Ken Behrens Annotated bird list by Jerry Connolly TOUR SUMMARY Madagascar has long been a core destination for Tropical Birding, and with the opening of a satellite office in the country several years ago, we further solidified our expertise in the “Eighth Continent.” This custom trip followed an itinerary similar to that of our main set-departure tour. Although this trip had a definite bird bias, it was really a general natural history tour. We took our time in observing and photographing whatever we could find, from lemurs to chameleons to bizarre invertebrates. Madagascar is rich in wonderful birds, and we enjoyed these to the fullest. But its mammals, reptiles, amphibians, and insects are just as wondrous and accessible, and a trip that ignored them would be sorely missing out. We also took time to enjoy the cultural riches of Madagascar, the small villages full of smiling children, the zebu carts which seem straight out of the Middle Ages, and the ingeniously engineered rice paddies. If you want to come to Madagascar and see it all… come with Tropical Birding! Madagascar is well known to pose some logistical challenges, especially in the form of the national airline Air Madagascar, but we enjoyed perfectly smooth sailing on this tour. We stayed in the most comfortable hotels available at each stop on the itinerary, including some that have just recently opened, and savored some remarkably good food, which many people rank as the best Madagascar Custom Tour October 20-November 6, 2016 they have ever had on any birding tour. -

Parson's Chameleon
L’ARCHE LES ÉCODOCS PHOTOGRAPHIQUE endangered species… ACTIONS FOR BIODIVERSITY PARSON’S CHAMELEON CHARACTERISTICS Kingdom Animal he chameleon is the unchallenged Phylum Chordata master of camouflage, and Parson’s Class Reptilia chameleon is by far the largest spe- Order Squamata cimen in Madagascar and on the Family Chamæleonidæ Tplanet! The endemic species lives only in the tro- Genus Calumma pical forests in the east of the Red Island. It can Latin name Calumma parsonii be easily recognised by its size. The adult has two Weight up to 1 kg outgrowths at the end of its muzzle, and the back Size 90 cm of its head extends in the shape of a helmet. Its Incubation period 21 months Number of eggs 20-25 limbs are stronger and more robust than those Interval between reproduction 2 years of all its fellows. It uses its small horns when it Diet Insectivore fights other males for a female. Longevity 4 years CITES Annexe II PAGE 1/3 LES ÉCODOCS PARSON’S endangered species… CHAMELEON Parson’s chameleon (Calumma parsonii) on the look-out. During a fight, the adversaries change from tur- that gives it a perfect estimate of its distance. Like quoise blue to threatening red. The vanquished a sucker, it catches its prey with the muscled tip male darkens his colours and goes flat on its of its large tongue covered with sticky saliva. Its belly under the branch. Conversely, the victor puts on even more flamboyant colours. The female couples only once every two years. Oviparous, she digs the soil in which to lay her eggs. -

Furcifer Cephalolepis Günther, 1880
AC22 Doc. 10.2 Annex 7 Furcifer cephalolepis Günther, 1880 FAMILY: Chamaeleonidae COMMON NAMES: Comoro Islands Chameleon (English); Caméléon des Comores (French) GLOBAL CONSERVATION STATUS: Not yet assessed by IUCN. SIGNIFICANT TRADE REVIEW FOR: Comoros Range State selected for review Range State Exports* Urgent, Comments (1994-2003) possible or least concern Comoros 7,150 Least Locally abundant. No trade recorded since 1993, when only 300 exported. concern No known monitoring or evidence of non-detriment findings. *Excluding re-exports SUMMARY Furcifer cephalolepis is a relatively small chameleon endemic to the island of Grand Comoro (Ngazidja) in the Comoros, where it occurs at altitudes of between 300 m and 650 m, and has an area of occupancy of between 300 km2 and 400 km2. It occurs in disturbed and secondary vegetation, including in towns, and can reportedly be locally abundant, although no quantitative measures of population size are available. Plausible estimates indicate that populations may be in the range of tens of thousands to hundreds of thousands. The species is exported as a live animal for the pet trade. Recorded exports from the Comoros began in 2000 and, between then and 2003, some 7,000 animals were recorded as exported, latterly almost all to the USA. Only 300 were recorded in trade in 2003, and none in 2004 (or, to date, in 2005), despite the fact that exports of other Comorean reptiles, which dropped to a low or zero level in 2003, began again in 2004. Captive breeding has taken place, in the USA at least. The species is not known to be covered by any national legislation. -

Madagascar: the Red Island
Andrea L. Baden & Rachel L. Jacobs Stony Brook University Taxonomic group Total species Endemic species % Endemism Plants 13,000 11,600 89.2 Mammals 155 144 92.9 Birds 310 181 58.4 Reptiles 384 367 95.6 Amphibians 230 229 99.6 Freshwater fish 164 97 59.1 *Recently extinct species: 45 (including birds, reptiles, and mammals) “The ecological state of being unique to a particular geographic location, such as a specific island…[Endemic species are] only found in that part of the world and nowhere else.” Taxonomic group Total species Endemic species % Endemism Plants 13,000 11,600 89.2 Mammals 155 144 92.9 Birds 310 181 58.4 Reptiles 384 367 95.6 Amphibians 230 229 99.6 Freshwater fish 164 97 59.1 *Recently extinct species: 45 (including birds, reptiles, and mammals) North & Central America . Phillippenes . California floristic province . Polynesia-Micronesia . Caribbean Islands . Southwest Australia . Madrean Pine Oak Woodlands . Sundaland . Mesoamerica . Wallaceae South America . Western Ghats & Sri Lanka . Atlantic Forest Europe & Central Asia . Cerrado . Caucasus . Chilean winter-Rainfall-Valdivian . Irano-Antalian forests . Mediterranean Basin . Tumbes-Choco-Magdalena . Mtns of Central Asia . Tropical Andes Africa Asia-Pacific . Cape Floristic region . E. Melanesian Islands . E. African coastal forests . Himalaya . Eastern afromontane . Indo-Burma . W. African Guinean forests . Japan . Horn of Africa . Mtns of SW China . Madagascar . New Caledonia . Maputaland-Pondoland-Albany . New Zealand . Succulent Karoo > 44% of the world’s plant species > 35% of the world’s terrestrial vertebrates Cover ~ 1.4% of the earth’s surface . was once 11%, but 88% of that has since been lost Madagascar contains 1 of 6 major radiations of primates . -

A Revision of the Chameleon Species Chamaeleo Pfeili Schleich
A revision of the chameleon species Chamaeleo pfeili Schleich (Squamata; Chamaeleonidae) with description of a new material of chamaeleonids from the Miocene deposits of southern Germany ANDREJ ÈERÒANSKÝ A revision of Chamaeleo pfeili Schleich is presented. The comparisons of the holotypic incomplete right maxilla with those of new specimens described here from the locality Langenau (MN 4b) and of the Recent species of Chamaeleo, Furcifer and Calumma is carried out. It is shown that the type material of C. pfeili and the material described here lack autapomorphic features. Schleich based his new species on the weak radial striations on the apical parts of bigger teeth. However, this character is seen in many species of extant chameleons, e.g. Calumma globifer, Furcifer pardalis and C. chamaeleon. For this reason, the name C. pfeili is considered a nomen dubium. This paper provides detailed descrip- tions and taxonomy of unpublished material from Petersbuch 2 (MN 4a) and Wannenwaldtobel (MN 5/6) in Germany. The material is only fragmentary and includes jaw bits. The morphology of the Petersbuch 2 material is very similar to that of the chameleons described from the Czech Republic. • Key words: Chamaeleo pfeili, nomen dubium, morphology, Wannenwaldtobel, Petersbuch 2, Langenau, Neogene. ČERŇANSKÝ, A. 2011. A revision of the chameleon species Chamaeleo pfeili Schleich (Squamata; Chamaeleonidae) with description of a new material of chamaeleonids from the Miocene deposits of southern Germany. Bulletin of Geosciences 86(2), 275–282 (6 figures). Czech Geological Survey, Prague. ISSN 1214-1119. Manuscript received Feb- ruary 11, 2011; accepted in revised form March 21, 2011; published online April 20, 2011; issued June 20, 2011. -

The Herpetological Journal
Volume 11, Number 2 April 2001 ISSN 0268-0130 THE HERPETOLOGICAL JOURNAL Published by the Indexed in BRITISH HERPETOLOGICAL SOCIETY Current Contents HERPETOLOGICAL JOURNAL, Vol. 11, pp. 53-68 (2001) TWO NEW CHAMELEONS OF THE GENUS CA L UMMA FROM NORTH-EAST MADAGASCAR, WITH OBSERVATIONS ON HEMIPENIAL MORPHOLOGY IN THE CA LUMMA FURCIFER GROUP (REPTILIA, SQUAMATA, CHAMAELEONIDAE) FRANCO ANDREONE1, FABIO MATTIOLI 2.3, RICCARDO JESU2 AND JASMIN E. RANDRIANIRINA4 1 Sezione di Zoologia, Museo Regionale di Scienze Natura/i, Zoological Department (Laboratory of Vertebrate Taxonomy and Ecology) , Via G. Giolitti, 36, I- 10123 Torino, Italy 1 Acquario di Genova, Area Porto Antico, Ponte Spinola, 1- 16128 Genova, Italy 3 Un iversity of Genoa, DIP. TE. RIS. , Zoology, Corso Europa, 26, 1- 16100 Genova, Italy 4 Pare Botanique et Zoologique de Tsimbazaza, Departement Fazme, BP 4096, Antananarivo (JOI), Madagascar During herpetological surveys in N. E. Madagascar two new species of Calumma chameleons belonging to the C. furcife r group were foundand are described here. The first species, Calumma vencesi n. sp., was found at three rainforest sites: Ambolokopatrika (corridor between the Anjanaharibe-Sud and Marojejy massifs), Besariaka (classifiedforest southof the Anjanaharibe Sud Massif), and Tsararano (forest between Besariaka and Masoala). This species is related to C. gastrotaenia, C. gui//aumeti and C: marojezensis. C. vencesi n. sp. differs in having a larger size, a dorsal crest, and - in fe males - a typical green coloration with a network of alternating dark and light semicircular stripes. Furthermore, it is characterized by a unique combination of hemipenis characters: a pair of sulcal rotulae anteriorly bearing a papillary fi eld; a pair of asulcal rotulae showing a double denticulated edge; and a pair of long pointed cylindrical papillae bearing a micropapillary field on top. -
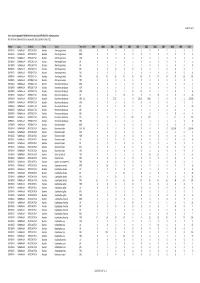
Gross Trade in Appendix II FAUNA (Direct Trade Only), 1999-2010 (For
AC25 Inf. 5 (1) Gross trade in Appendix II FAUNA (direct trade only), 1999‐2010 (for selection process) N.B. Data from 2009 and 2010 are incomplete. Data extracted 1 April 2011 Phylum Class TaxOrder Family Taxon Term Unit 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Total CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BOD 0 00001000102 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BON 0 00080000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia HOR 0 00000110406 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia LIV 0 00060000006 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKI 1 11311000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKP 0 00000010001 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKU 2 052101000011 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia TRO 15 42 49 43 46 46 27 27 14 37 26 372 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Antilope cervicapra TRO 0 00000020002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae BOD 0 00100001002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOP 0 00200000002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOR 0 0010100120216 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae LIV 0 0 5 14 0 0 0 30 0 0 0 49 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA KIL 0 5 27.22 0 0 272.16 1000 00001304.38 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA 0 00000000101 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae -

Description of a New Pygmy Chameleon (Chamaeleonidae: Brookesia) from Central Madagascar
Zootaxa 3490: 63–74 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2012 · Magnolia Press Article ISSN 1175-5334 (online edition) urn:lsid:zoobank.org:pub:FF22F75B-4A07-40D9-9609-1B8D269A921C Description of a new pygmy chameleon (Chamaeleonidae: Brookesia) from central Madagascar ANGELICA CROTTINI1,2,5, AURÉLIEN MIRALLES2, FRANK GLAW3, D. JAMES HARRIS1, ALEXANDRA LIMA1,4 & MIGUEL VENCES2 1CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Campus Agrário de Vairão, R. Padre Armando Quintas, 4485-661 Vairão, Portugal. E-mail: [email protected] 2Zoological Institute, Division of Evolutionary Biology, Technical University of Braunschweig, Mendelssohnstraße 4, 38106 Braunschweig, Germany 3Zoologische Staatssammlung München, Münchhausenstr. 21, 81247 München, Germany 4Departamento de Biologia, Faculdade de Ciências da Universidade do Porto, R. Campo Alegre s/n, 4169-007 Porto, Portugal 5Corresponding author Abstract We describe a new Brookesia species from a forest fragment located 13 km south of Ambalavao in the southern part of Madagascar's central high plateau. Brookesia brunoi sp. nov. is one of the few arid-adapted Brookesia species inhabiting deciduous forests on the western slope of the central high plateau of the island (around 950 m a.s.l.). So far the species has only been observed in the private Anja Reserve. The species belongs to the Brookesia decaryi group formed by arid-adapt- ed Brookesia species of western Madagascar: B. bonsi Ramanantsoa, B. perarmata (Angel), B. brygooi Raxworthy & Nussbaum and B. decaryi Angel. Brookesia brunoi differs from the other four species of the group by a genetic divergence of more than 17.6% in the mitochondrial ND2 gene, and by a combination of morphological characters: (1) nine pairs of laterovertebral pointed tubercles, (2) absence of enlarged pointed tubercles around the vent, (3) presence of poorly defined laterovertebral tubercles along the entire tail, (4) by the configuration of its cephalic crest, and (5) hemipenial morphology. -

COMMISSION IMPLEMENTING REGULATION (EU) 2017/1915 Of
20.10.2017 EN Official Journal of the European Union L 271/7 COMMISSION IMPLEMENTING REGULATION (EU) 2017/1915 of 19 October 2017 prohibiting the introduction into the Union of specimens of certain species of wild fauna and flora THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Council Regulation (EC) No 338/97 of 9 December 1996 on the protection of species of wild fauna and flora by regulating trade therein (1), and in particular Article 4(6) thereof, Whereas: (1) The purpose of Regulation (EC) No 338/97 is to protect species of wild fauna and flora and to guarantee their conservation by regulating trade in animal and plant species listed in its Annexes. The species listed in the Annexes include the species set out in the Appendices to the Convention on International Trade in Endangered Species of Wild Fauna and Flora signed in 1973 (2) (the Convention) as well as species whose conservation status requires that trade from, into and within the Union be regulated or monitored. (2) Article 4(6) of Regulation (EC) No 338/97 provides that the Commission may establish restrictions to the introduction of specimens of certain species into the Union in accordance with the conditions laid down in points (a) to (d) thereof. (3) On the basis of recent information, the Scientific Review Group established pursuant to Article 17 of Regulation (EC) No 338/97 has concluded that the conservation status of certain species listed in Annex B to Regulation (EC) No 338/97 would be seriously jeopardised if their introduction into the Union from certain countries of origin is not prohibited. -

Extending the Known Distribution of Nicosia's Chameleon
Herpetology Notes, volume 14: 455-460 (2021) (published online on 26 February 2021) Extending the known distribution of Nicosia’s chameleon, Furcifer nicosiai Jesu, Mattioli & Schimmenti, 1999 (Squamata: Chamaeleonidae) Francesco Belluardo1,*, Gonçalo M. Rosa2,3, Franco Andreone4, Elodie A. Courtois5, Javier Lobón-Rovira1, Ronald A. Nussbaum6, Miary Raselimanana7, Malalatiana Rasoazanany7, Christopher J. Raxworthy8, and Angelica Crottini1 The genus Furcifer Fitzinger, 1843 includes 24 region (Fig. 1, white circles; Table 1) (Randrianantoandro species of chameleons, most of which are endemic to et al., 2008; Raselimanana, 2008; Bora et al., 2010; Madagascar (Glaw and Vences, 2007; Uetz et al., 2020). Randriamoria, 2011; Brown et al., 2014; Goodman et Furcifer nicosiai Jesu, Mattioli & Schimmenti, 1999 al., 2018). Furcifer nicosiai habitat encompasses dense is a medium-sized species belonging to the Furcifer sub-humid and dry forests of low elevation, between verrucosus (Cuvier, 1829) phenetic group (Glaw and 57–571 m above sea level ~ a.s.l. (Bora et al., 2010). Vences, 2007). Although slightly smaller, F. nicosiai is Several records within the Menabe region (within the morphologically similar to Furcifer oustaleti (Mocquard, Paysage Harmonieux Protégé de Menabe Antimena, 1894), whose subadults can be mistaken with adults of about 60 km south of Tsingy de Bemaraha) refer to this species (Glaw and Vences, 2007). a population of F. nicosiai that appears to have some With Tsingy de Bemaraha as the type locality of F. morphological differences to the population from the nicosiai (Jesu et al., 1999), the species was thought to type locality. A molecular characterisation is needed have a distribution limited to western Madagascar, with, to assess the taxonomic identity of these populations, until now, only a few additional records in the Melaky but for consistency we here continue to assign them to this species (Raselimanana, 2008; Randrianantoandro et al., 2010; Eckhardt et al., 2019) (Fig.