Systematic Revision of the Malagasy Chameleons Calumma Boettgeri and C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MADAGASCAR: the Wonders of the “8Th Continent” a Tropical Birding Custom Trip
MADAGASCAR: The Wonders of the “8th Continent” A Tropical Birding Custom Trip October 20—November 6, 2016 Guide: Ken Behrens All photos taken during this trip by Ken Behrens Annotated bird list by Jerry Connolly TOUR SUMMARY Madagascar has long been a core destination for Tropical Birding, and with the opening of a satellite office in the country several years ago, we further solidified our expertise in the “Eighth Continent.” This custom trip followed an itinerary similar to that of our main set-departure tour. Although this trip had a definite bird bias, it was really a general natural history tour. We took our time in observing and photographing whatever we could find, from lemurs to chameleons to bizarre invertebrates. Madagascar is rich in wonderful birds, and we enjoyed these to the fullest. But its mammals, reptiles, amphibians, and insects are just as wondrous and accessible, and a trip that ignored them would be sorely missing out. We also took time to enjoy the cultural riches of Madagascar, the small villages full of smiling children, the zebu carts which seem straight out of the Middle Ages, and the ingeniously engineered rice paddies. If you want to come to Madagascar and see it all… come with Tropical Birding! Madagascar is well known to pose some logistical challenges, especially in the form of the national airline Air Madagascar, but we enjoyed perfectly smooth sailing on this tour. We stayed in the most comfortable hotels available at each stop on the itinerary, including some that have just recently opened, and savored some remarkably good food, which many people rank as the best Madagascar Custom Tour October 20-November 6, 2016 they have ever had on any birding tour. -

Parson's Chameleon
L’ARCHE LES ÉCODOCS PHOTOGRAPHIQUE endangered species… ACTIONS FOR BIODIVERSITY PARSON’S CHAMELEON CHARACTERISTICS Kingdom Animal he chameleon is the unchallenged Phylum Chordata master of camouflage, and Parson’s Class Reptilia chameleon is by far the largest spe- Order Squamata cimen in Madagascar and on the Family Chamæleonidæ Tplanet! The endemic species lives only in the tro- Genus Calumma pical forests in the east of the Red Island. It can Latin name Calumma parsonii be easily recognised by its size. The adult has two Weight up to 1 kg outgrowths at the end of its muzzle, and the back Size 90 cm of its head extends in the shape of a helmet. Its Incubation period 21 months Number of eggs 20-25 limbs are stronger and more robust than those Interval between reproduction 2 years of all its fellows. It uses its small horns when it Diet Insectivore fights other males for a female. Longevity 4 years CITES Annexe II PAGE 1/3 LES ÉCODOCS PARSON’S endangered species… CHAMELEON Parson’s chameleon (Calumma parsonii) on the look-out. During a fight, the adversaries change from tur- that gives it a perfect estimate of its distance. Like quoise blue to threatening red. The vanquished a sucker, it catches its prey with the muscled tip male darkens his colours and goes flat on its of its large tongue covered with sticky saliva. Its belly under the branch. Conversely, the victor puts on even more flamboyant colours. The female couples only once every two years. Oviparous, she digs the soil in which to lay her eggs. -

A Revision of the Chameleon Species Chamaeleo Pfeili Schleich
A revision of the chameleon species Chamaeleo pfeili Schleich (Squamata; Chamaeleonidae) with description of a new material of chamaeleonids from the Miocene deposits of southern Germany ANDREJ ÈERÒANSKÝ A revision of Chamaeleo pfeili Schleich is presented. The comparisons of the holotypic incomplete right maxilla with those of new specimens described here from the locality Langenau (MN 4b) and of the Recent species of Chamaeleo, Furcifer and Calumma is carried out. It is shown that the type material of C. pfeili and the material described here lack autapomorphic features. Schleich based his new species on the weak radial striations on the apical parts of bigger teeth. However, this character is seen in many species of extant chameleons, e.g. Calumma globifer, Furcifer pardalis and C. chamaeleon. For this reason, the name C. pfeili is considered a nomen dubium. This paper provides detailed descrip- tions and taxonomy of unpublished material from Petersbuch 2 (MN 4a) and Wannenwaldtobel (MN 5/6) in Germany. The material is only fragmentary and includes jaw bits. The morphology of the Petersbuch 2 material is very similar to that of the chameleons described from the Czech Republic. • Key words: Chamaeleo pfeili, nomen dubium, morphology, Wannenwaldtobel, Petersbuch 2, Langenau, Neogene. ČERŇANSKÝ, A. 2011. A revision of the chameleon species Chamaeleo pfeili Schleich (Squamata; Chamaeleonidae) with description of a new material of chamaeleonids from the Miocene deposits of southern Germany. Bulletin of Geosciences 86(2), 275–282 (6 figures). Czech Geological Survey, Prague. ISSN 1214-1119. Manuscript received Feb- ruary 11, 2011; accepted in revised form March 21, 2011; published online April 20, 2011; issued June 20, 2011. -
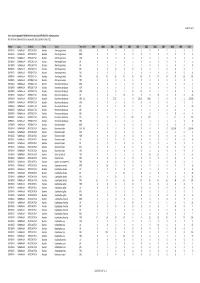
Gross Trade in Appendix II FAUNA (Direct Trade Only), 1999-2010 (For
AC25 Inf. 5 (1) Gross trade in Appendix II FAUNA (direct trade only), 1999‐2010 (for selection process) N.B. Data from 2009 and 2010 are incomplete. Data extracted 1 April 2011 Phylum Class TaxOrder Family Taxon Term Unit 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Total CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BOD 0 00001000102 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BON 0 00080000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia HOR 0 00000110406 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia LIV 0 00060000006 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKI 1 11311000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKP 0 00000010001 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKU 2 052101000011 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia TRO 15 42 49 43 46 46 27 27 14 37 26 372 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Antilope cervicapra TRO 0 00000020002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae BOD 0 00100001002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOP 0 00200000002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOR 0 0010100120216 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae LIV 0 0 5 14 0 0 0 30 0 0 0 49 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA KIL 0 5 27.22 0 0 272.16 1000 00001304.38 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA 0 00000000101 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae -

COMMISSION IMPLEMENTING REGULATION (EU) 2017/1915 Of
20.10.2017 EN Official Journal of the European Union L 271/7 COMMISSION IMPLEMENTING REGULATION (EU) 2017/1915 of 19 October 2017 prohibiting the introduction into the Union of specimens of certain species of wild fauna and flora THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Council Regulation (EC) No 338/97 of 9 December 1996 on the protection of species of wild fauna and flora by regulating trade therein (1), and in particular Article 4(6) thereof, Whereas: (1) The purpose of Regulation (EC) No 338/97 is to protect species of wild fauna and flora and to guarantee their conservation by regulating trade in animal and plant species listed in its Annexes. The species listed in the Annexes include the species set out in the Appendices to the Convention on International Trade in Endangered Species of Wild Fauna and Flora signed in 1973 (2) (the Convention) as well as species whose conservation status requires that trade from, into and within the Union be regulated or monitored. (2) Article 4(6) of Regulation (EC) No 338/97 provides that the Commission may establish restrictions to the introduction of specimens of certain species into the Union in accordance with the conditions laid down in points (a) to (d) thereof. (3) On the basis of recent information, the Scientific Review Group established pursuant to Article 17 of Regulation (EC) No 338/97 has concluded that the conservation status of certain species listed in Annex B to Regulation (EC) No 338/97 would be seriously jeopardised if their introduction into the Union from certain countries of origin is not prohibited. -

An Observation of Calumma Tigris (Squamata: Chamaeleonidae) Feeding on White-Footed Ants, Technomyrmex Albipes Complex, in the Seychelles
An observation of Calumma tigris (Squamata: Chamaeleonidae) feeding on White-footed ants, Technomyrmex albipes complex, in the Seychelles HENRIK BRINGSOE Irisvej 8, DK-4600 Koge, Denmark. E-mail: [email protected] ABSTRACT — A sub-dult Calumma tigris was observed eating White-footed ants, Technomynnex albipes complex, in the Vallee de Mai national park on Praslin (Seychelles). It is noteworthy that this species of ant is, as far as can be demonstrated, not eaten by any other predator in the Seychelles. Considering the restricted range and possibly low population densities of C. tigris, it will be relevant to investigate its natural diet. It will also be crucial to monitor the role of White-footed ants in Seychellois ecosystems which are fragile and very sensitive to allochthonous species. HE biology of the Tiger chameleon, Calumma spots or some brighter coloured markings. Ttigris (Kuhl, 1820), of the Seychelles is not Generally a light coloured vertebral line is present. well known. Merely a little anecdotal information An interesting observation on colour change has has been published and some scattered reports on been expressed by van Heygen & van Heygen reproduction exist. Thus, until proper studies will (2004). They state that the colours of C. tigris be carried out, virtually any contribution to the depend on the environment, i.e. crypsis as the biology of C. tigris may prove valuable. individuals resemble a random sample of the Calumna tigris is a relatively small species relevant aspects of their environment. That would which normally attains a total length of approx 16 be unusual for chameleons (Neeas, 2004a, 2004b)! cm. -

Final Report
Darwin Initiative – Final Report Darwin project information Project Reference 17-010 Project Title Chameleon trade and conservation in Madagascar Host country(ies) Madagascar UK Contract Holder Durrell Institute of Conservation and Ecology (DICE) UK Partner Institution(s) None Host Country Partner Madagasikara Voakajy Darwin Grant Value £XXX Start/End dates of Project 1 May 2009 to 30 April 2012 Project Leader Name Richard Griffiths Project Website www.madagasikara-voakajy.org https://www.facebook.com/pages/Madagasikara- Voakajy/310507418994236 Report Author(s) and date Richard Jenkins, Christian Randrianantoandro, Richard Griffiths 1 Project Background The island of Madagascar is home to over 83 species of endemic chameleons. These unique lizards, admired by the island’s tourists but disliked by many of its residents, occur in all habitats from sea-level to the highest mountains. However, chameleons in Madagascar are subject to a wide array of threats, including habitat loss, illegal collection and climate change. The three outstanding achievements of this project were: 1) Assessed the conservation status of 76 Malagasy chameleons for the IUCN Red List of Threatened Species 2) Completed a survey of illegal trade in Malagasy chameleons in Thailand 3) Provided essential support to the CITES authorities in Madagascar that led to Standing Committee’s lifting of the trade suspension for some species after 18 years, and an export quota for Furcifer campani 2 Project support to the Convention on Biological Diversity (CBD) (a) This project mainly contributed to two of the Focal Areas in the CBD 2010 Biodiversity Targets. It promoted the conservation of species diversity (Goal 2) by identifying chameleons at highest risk of extinction and then designing and implementing actions to improve the status of these species (Targets 2.1 & 2.2). -

'True Chameleon' from Madagascar: a New, Distinctly Colored
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Zoosystematics and Evolution Jahr/Year: 2018 Band/Volume: 94 Autor(en)/Author(s): Prötzel David, Lambert Shea M., Andrianasolo Ginah Tsiorisoa, Hutter Carl R., Cobb Kerry A., Scherz Mark D., Glaw Frank Artikel/Article: The smallest ‘true chameleon’ from Madagascar: a new, distinctly colored species of the Calumma boettgeri complex (Squamata, Chamaeleonidae) 409-423 Creative Commons Attribution 4.0 licence (CC-BY); original download https://pensoft.net/journals Zoosyst. Evol. 94 (2) 2018, 409–423 | DOI 10.3897/zse.94.27305 The smallest ‘true chameleon’ from Madagascar: a new, distinctly colored species of the Calumma boettgeri complex (Squamata, Chamaeleonidae) David Prötzel1, Shea M. Lambert2, Ginah Tsiorisoa Andrianasolo3, Carl R. Hutter4, Kerry A. Cobb5, Mark D. Scherz1,6, Frank Glaw1 1 Zoologische Staatssammlung München (ZSM-SNSB), Münchhausenstr. 21, 81247 München, Germany 2 Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA 3 Mention Zoologie et Biodiversité Animale, Université d’Antananarivo, BP 906, Antananarivo 101, Madagascar 4 Biodiversity Institute and Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, KS 66045–7561, USA 5 Department of Biological Sciences, Auburn University, Auburn, AL 36849, USA 6 Zoologisches Institut, Technische Universität Braunschweig, Mendelssohnstr. 4, 38106 Braunschweig, Germany http://zoobank.org/2433A9DD-8AC1-4139-A639-E24053D5C33F Corresponding author: David Prötzel ([email protected]) Abstract Received 8 June 2018 On a recent expedition to eastern Madagascar, we discovered a distinct new species of the Accepted 10 August 2018 genus Calumma that we describe here using an integrative approach combining morphol- Published 19 October 2018 ogy, coloration, osteology and molecular genetics. -

Calumma Vohibola, a New Chameleon Species (Squamata: Chamaeleonidae) from the Littoral Forests of Eastern Madagascar Philip-Sebastian Gehring a , Fanomezana M
This article was downloaded by: [Sebastian Gehring] On: 26 October 2011, At: 23:51 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK African Journal of Herpetology Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/ther20 Calumma vohibola, a new chameleon species (Squamata: Chamaeleonidae) from the littoral forests of eastern Madagascar Philip-Sebastian Gehring a , Fanomezana M. Ratsoavina a b c , Miguel Vences a & Frank Glaw d a Division of Evolutionary Biology, Zoological Institute, Technical University of Braunschweig, Mendelssohnstr. 4, 38106, Braunschweig, Germany b Département de Biologie Animale, Université d'Antananarivo, BP 906, Antananarivo, 101, Madagascar c Grewcock Center for Conservation Research, Omaha's Henry Doorly Zoo, 3701 South 10th Street, Omaha, NE, 68107-2200, USA d Zoologische Staatssammlung München, Münchhausenstr. 21, 81247, München, Germany Available online: 26 Oct 2011 To cite this article: Philip-Sebastian Gehring, Fanomezana M. Ratsoavina, Miguel Vences & Frank Glaw (2011): Calumma vohibola, a new chameleon species (Squamata: Chamaeleonidae) from the littoral forests of eastern Madagascar, African Journal of Herpetology, 60:2, 130-154 To link to this article: http://dx.doi.org/10.1080/21564574.2011.628412 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.tandfonline.com/page/terms-and- conditions This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. -

Companion Reptile Care SERIES the Veiled Chameleon
cham.qxd 8/10/2007 3:43 PM Page 1 VEILED Most Common Disorders The veiled chameleon of Veiled Chameleons (Chamaeleo calyptratus) is a •Dystocia (egg-binding) large, colorful, and robust lizard •Metabolic bone disease indigenous to coastal regions of CHAMELEON •Toenail loss / foot infections Yemen and Saudi Arabia. Now well •Intestinal parasites established in captivity, it is one •Respiratory / sinus / ocular infections of the most popular and widely •Stomatitis / periodontal disease recommended chameleons for •Abscesses / cellulitis / osteomyelitis the novice reptile keeper. •Loss of tongue function •Kidney disease A characteristic feature of this •Hemipene prolapse species is the impressively high •Dehydration casque on the head. Adult males have a higher casque than females. Having your veiled chameleon examined on a regular basis Some authorities have suggested that by an exotic animal veterinarian who is familiar with the casque may serve to collect reptiles can prevent many of the common disorders above. and channel water, such as morning dewdrops or fog, into the mouth. Others believe that it functions to dissipate heat. A more recent hypothesis suggests that it may amplify a low frequency “buzzing” used by this species to communicate with one another. Veiled chameleons also possess prehensile tails, long whip-like tongues, independently moving eyes, zygodactyl feet, and a spectacular array of changing colors. Zoological Education Network provides educational ©2007 Zoological Education Network materials about exotic companion animals. 800-946-4782 561-641-6745 www.exoticdvm.com CompanionC Reptile Care SERIES cham.qxd 8/10/2007 3:43 PM Page 2 What Your Veterinarian Looks for in a Healthy Veiled Chameleon Active and alert What to Expect from Your Veiled Chameleon Eyes open and clear attitude hameleons are unique, attractive and fascinating 24 hours before feeding them out. -

No Longer Single! Description of Female Calumma Vatosoa (Squamata, Chamaeleonidae) Including a Review of the Species and Its Systematic Position
Zoosyst. Evol. 92 (1) 2016, 13–21 | DOI 10.3897/zse.92.6464 museum für naturkunde No longer single! Description of female Calumma vatosoa (Squamata, Chamaeleonidae) including a review of the species and its systematic position David Prötzel1, Bernhard Ruthensteiner1, Frank Glaw1 1 Zoologische Staatssammlung München (ZSM-SNSB), Münchhausenstr. 21, 81247 München, Germany http://zoobank.org/CFD64DFB-D085-4D1A-9AA9-1916DB6B4043 Corresponding author: David Prötzel ([email protected]) Abstract Received 3 September 2015 Calumma vatosoa is a Malagasy chameleon species that has until now been known only Accepted 26 November 2015 from the male holotype and a photograph of an additional male specimen. In this paper Published 8 January 2016 we describe females of the chameleon Calumma vatosoa for the first time, as well as the skull osteology of this species. The analysed females were collected many years before Academic editor: the description of C. vatosoa, and were originally described as female C. linotum. Ac- Johannes Penner cording to external morphology, osteology, and distribution these specimens are assigned to C. vatosoa. Furthermore we discuss the species group assignment of C. vatosoa and transfer it from the C. furcifer group to the C. nasutum group. A comparison of the exter- Key Words nal morphology of species of both groups revealed that C. vatosoa has a relatively shorter distance from the anterior margin of the orbit to the snout tip, more heterogeneous scala- Madagascar tion at the lower arm, a significantly lower number of supralabial and infralabial scales, chameleon and a relatively longer tail than the members of the C. furcifer group. -

The New Mode of Thought of Vertebrates' Evolution
etics & E en vo g lu t lo i y o h n a P r f y Journal of Phylogenetics & Kupriyanova and Ryskov, J Phylogen Evolution Biol 2014, 2:2 o B l i a o n l r o DOI: 10.4172/2329-9002.1000129 u g o y J Evolutionary Biology ISSN: 2329-9002 Short Communication Open Access The New Mode of Thought of Vertebrates’ Evolution Kupriyanova NS* and Ryskov AP The Institute of Gene Biology RAS, 34/5, Vavilov Str. Moscow, Russia Abstract Molecular phylogeny of the reptiles does not accept the basal split of squamates into Iguania and Scleroglossa that is in conflict with morphological evidence. The classical phylogeny of living reptiles places turtles at the base of the tree. Analyses of mitochondrial DNA and nuclear genes join crocodilians with turtles and places squamates at the base of the tree. Alignment of the reptiles’ ITS2s with the ITS2 of chordates has shown a high extent of their similarity in ancient conservative regions with Cephalochordate Branchiostoma floridae, and a less extent of similarity with two Tunicata, Saussurea tunicate, and Rinodina tunicate. We have performed also an alignment of ITS2 segments between the two break points coming into play in 5.8S rRNA maturation of Branchiostoma floridaein pairs with orthologs from different vertebrates where it was possible. A similarity for most taxons fluctuates between about 50 and 70%. This molecular analysis coupled with analysis of phylogenetic trees constructed on a basis of manual alignment, allows us to hypothesize that primitive chordates being the nearest relatives of simplest vertebrates represent the real base of the vertebrate phylogenetic tree.