Canning Wild Mushrooms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Field Guide to Common Macrofungi in Eastern Forests and Their Ecosystem Functions
United States Department of Field Guide to Agriculture Common Macrofungi Forest Service in Eastern Forests Northern Research Station and Their Ecosystem General Technical Report NRS-79 Functions Michael E. Ostry Neil A. Anderson Joseph G. O’Brien Cover Photos Front: Morel, Morchella esculenta. Photo by Neil A. Anderson, University of Minnesota. Back: Bear’s Head Tooth, Hericium coralloides. Photo by Michael E. Ostry, U.S. Forest Service. The Authors MICHAEL E. OSTRY, research plant pathologist, U.S. Forest Service, Northern Research Station, St. Paul, MN NEIL A. ANDERSON, professor emeritus, University of Minnesota, Department of Plant Pathology, St. Paul, MN JOSEPH G. O’BRIEN, plant pathologist, U.S. Forest Service, Forest Health Protection, St. Paul, MN Manuscript received for publication 23 April 2010 Published by: For additional copies: U.S. FOREST SERVICE U.S. Forest Service 11 CAMPUS BLVD SUITE 200 Publications Distribution NEWTOWN SQUARE PA 19073 359 Main Road Delaware, OH 43015-8640 April 2011 Fax: (740)368-0152 Visit our homepage at: http://www.nrs.fs.fed.us/ CONTENTS Introduction: About this Guide 1 Mushroom Basics 2 Aspen-Birch Ecosystem Mycorrhizal On the ground associated with tree roots Fly Agaric Amanita muscaria 8 Destroying Angel Amanita virosa, A. verna, A. bisporigera 9 The Omnipresent Laccaria Laccaria bicolor 10 Aspen Bolete Leccinum aurantiacum, L. insigne 11 Birch Bolete Leccinum scabrum 12 Saprophytic Litter and Wood Decay On wood Oyster Mushroom Pleurotus populinus (P. ostreatus) 13 Artist’s Conk Ganoderma applanatum -

SOMA News March 2011
VOLUME 23 ISSUE 7 March 2011 SOMA IS AN EDUCATIONAL ORGANIZATION DEDICATED TO MYCOLOGY. WE ENCOURAGE ENVIRONMENTAL AWARENESS BY SHARING OUR ENTHUSIASM THROUGH PUBLIC PARTICIPATION AND GUIDED FORAYS. WINTER/SPRING 2011 SPEAKER OF THE MONTH SEASON CALENDAR March Connie and Patrick March 17th » Meeting—7pm —“A Show and Tell”— Sonoma County Farm Bureau Speaker: Connie Green & Patrick March 17th—7pm Hamilton Foray March. 19th » Salt Point April April 21st » Meeting—7pm Sonoma County Farm Bureau Speaker: Langdon Cook Foray April 23rd » Salt Point May May 19th » Meeting—7pm Sonoma County Farm Bureau Speaker: Bob Cummings Foray May: Possible Morel Camping! eparated at birth but from the same litter Connie Green and Patrick Hamilton have S traveled (endured?) mushroom journeys together for almost two decades. They’ve been to the humid and hot jaguar jungles of Chiapas chasing tropical mushrooms and to EMERGENCY the cloud forests of the Sierra Madre for boletes and Indigo milky caps. In the cold and wet wilds of Alaska they hiked a spruce and hemlock forest trail to watch grizzly bears MUSHROOM tearing salmon bellies just a few yards away. POISONING IDENTIFICATION In the remote Queen Charlotte Islands their bush plane flew over “fields of golden chanterelles,” landed on the ocean, and then off into a zany Zodiac for a ride over a cold After seeking medical attention, contact and roiling sea alongside some low flying puffins to the World Heritage Site of Ninstints. Darvin DeShazer for identification at The two of them have gazed at glaciers and berry picked on muskeg bogs. More than a (707) 829-0596. -

Species List for Arizona Mushroom Society White Mountains Foray August 11-13, 2016
Species List for Arizona Mushroom Society White Mountains Foray August 11-13, 2016 **Agaricus sylvicola grp (woodland Agaricus, possibly A. chionodermus, slight yellowing, no bulb, almond odor) Agaricus semotus Albatrellus ovinus (orange brown frequently cracked cap, white pores) **Albatrellus sp. (smooth gray cap, tiny white pores) **Amanita muscaria supsp. flavivolvata (red cap with yellow warts) **Amanita muscaria var. guessowii aka Amanita chrysoblema (yellow cap with white warts) **Amanita “stannea” (tin cap grisette) **Amanita fulva grp.(tawny grisette, possibly A. “nishidae”) **Amanita gemmata grp. Amanita pantherina multisquamosa **Amanita rubescens grp. (all parts reddening) **Amanita section Amanita (ring and bulb, orange staining volval sac) Amanita section Caesare (prov. name Amanita cochiseana) Amanita section Lepidella (limbatulae) **Amanita section Vaginatae (golden grisette) Amanita umbrinolenta grp. (slender, ringed cap grisette) **Armillaria solidipes (honey mushroom) Artomyces pyxidatus (whitish coral on wood with crown tips) *Ascomycota (tiny, grayish/white granular cups on wood) **Auricularia Americana (wood ear) Auriscalpium vulgare Bisporella citrina (bright yellow cups on wood) Boletus barrowsii (white king bolete) Boletus edulis group Boletus rubriceps (red king bolete) Calyptella capula (white fairy lanterns on wood) **Cantharellus sp. (pink tinge to cap, possibly C. roseocanus) **Catathelesma imperiale Chalciporus piperatus Clavariadelphus ligula Clitocybe flavida aka Lepista flavida **Coltrichia sp. Coprinellus -

MUSHROOMS of the OTTAWA NATIONAL FOREST Compiled By
MUSHROOMS OF THE OTTAWA NATIONAL FOREST Compiled by Dana L. Richter, School of Forest Resources and Environmental Science, Michigan Technological University, Houghton, MI for Ottawa National Forest, Ironwood, MI March, 2011 Introduction There are many thousands of fungi in the Ottawa National Forest filling every possible niche imaginable. A remarkable feature of the fungi is that they are ubiquitous! The mushroom is the large spore-producing structure made by certain fungi. Only a relatively small number of all the fungi in the Ottawa forest ecosystem make mushrooms. Some are distinctive and easily identifiable, while others are cryptic and require microscopic and chemical analyses to accurately name. This is a list of some of the most common and obvious mushrooms that can be found in the Ottawa National Forest, including a few that are uncommon or relatively rare. The mushrooms considered here are within the phyla Ascomycetes – the morel and cup fungi, and Basidiomycetes – the toadstool and shelf-like fungi. There are perhaps 2000 to 3000 mushrooms in the Ottawa, and this is simply a guess, since many species have yet to be discovered or named. This number is based on lists of fungi compiled in areas such as the Huron Mountains of northern Michigan (Richter 2008) and in the state of Wisconsin (Parker 2006). The list contains 227 species from several authoritative sources and from the author’s experience teaching, studying and collecting mushrooms in the northern Great Lakes States for the past thirty years. Although comments on edibility of certain species are given, the author neither endorses nor encourages the eating of wild mushrooms except with extreme caution and with the awareness that some mushrooms may cause life-threatening illness or even death. -

Fall 2012 Species List Annex October 2012 Lummi Island Foray Species List
MushRumors The Newsletter of the Northwest Mushroomers Association Volume 23, Issue 2 Summer - Fall, 2012 Record 88 Days Without Measurable Precipitation Defines Northwest Washington’s 2012 Mushroom Season As June had faded into the first week of July, after a second consecutive year of far above average rainfall, no one could have anticipated that by the end of the second week of July we would see virtually no more rain until October 13th, only days before the Northwest Mushroomer’s Association annual Fall Mushroom Show. The early part of the season had offered up bumper crops of the prince (Agaricus augustus), and, a bit later, similar Photo by Jack Waytz quantities of the sulphur shelf (Laetiporus coniferarum). There were also some anomalous fruitings, which seemed completely inexplicable, such as matsutake mushrooms found in June and an exquisite fruiting of Boletus edulis var. grand edulis in Mt. Vernon at the end of the first week of July, under birch, (Pictured on page 2 of this letter) and Erin Moore ran across the king at the Nooksack in Deming, under western hemlock! As was the case last year, there was a very robust fruiting of lobster mushrooms in advance of the rains. It would seem that they need little, or no moisture at all, to have significant flushes. Apparently, a combination of other conditions, which remain hidden from the 3 princes, 3 pounds! mushroom hunters, herald their awakening. The effects of the sudden drought were predictably profound on the mycological landscape. I ventured out to Baker Lake on September 30th, and to my dismay, the luxuriant carpet of moss which normally supports a myriad of Photo by Jack Waytz species had the feel of astro turf, and I found not a single mushroom there, of any species. -

Big South Fork National River & Recreation Area Species List
Big South Fork National River & Recreation Area Species List Place cursor over cells with red By Cumberland Mycological Society, Crossville, TN triangles to view pictures Click underlined x's for photo links and/or comments click on underlined species for web links to details about those species Scientific name common names (if applicable) Sep-14 Edibility Notes* Akanthomyces aculeatus none x no information Amanita abrupta "Abrupt-bulbed Lepidella" x unknown and possibly poisonous Amanita americrocea syn. Amanitopsis crocea “Orange Grisette” x edible -with extreme caution!! Amanita amerifulva [often called 'Amanita fulva' -a European species] “Tawny Grisette” x edible -with extreme caution!! Amanita amerirubescens "Blusher" x edible -with extreme caution!! Amanita banningiana "Mary Banning's Slender Caesar" x no information Amanita bisporigera = A. virosa sensu auct. amer. (Ref. RET) "Destroying Angel" x deadly poisonous! Amanita brunnescens “Cleft foot-Amanita” x possibly poisonous Amanita citrina sensu auct. amer. "Citron Amanita," "False Death Cap" x possibly poisonous Amanita flavoconia “Yellow Patches" x possibly poisonous Amanita multisquamosa syn. A. pantherina, var. multisquamosa "Panther" x poisonous Armillaria caligata var. glaucescens none x edible, but most often bitter and smelly Austroboletus gracilis var. gracilis syn. Tylopilus gracilis “Graceful Bolete” x edible Boletus longicurvipes none x edible Boletus pallidus "Pale Bolete" x edible Callistosporium purpureomarginatum none x unknown Calostoma lutescens "Yellow Calostoma" x inedible Cantharellus appalachiensis "Appalachian Chanterelle" x edible Cantharellus "cibarius" "Golden Chanterelle" x choice edible -with caution Cantharellus cinnabarinus “Cinnabar Chanterelle” x edible and good Chrysomphalina chrysophylla syn. Gerronema chrysophylla "Golden-gilled Gerronema" x unknown Clitocybe subconnexa syn. Clitopilus caespitosus "Clustered Clitocybe" x not recommended Coltricia cinnamomea syn. -

How to Distinguish Amanita Smithiana from Matsutake and Catathelasma Species
VOLUME 57: 1 JANUARY-FEBRUARY 2017 www.namyco.org How to Distinguish Amanita smithiana from Matsutake and Catathelasma species By Michael W. Beug: Chair, NAMA Toxicology Committee A recent rash of mushroom poisonings involving liver failure in Oregon prompted Michael Beug to issue the following photos and information on distinguishing the differences between the toxic Amanita smithiana and edible Matsutake and Catathelasma. Distinguishing the choice edible Amanita smithiana Amanita smithiana Matsutake (Tricholoma magnivelare) from the highly poisonous Amanita smithiana is best done by laying the stipe (stem) of the mushroom in the palm of your hand and then squeezing down on the stipe with your thumb, applying as much pressure as you can. Amanita smithiana is very firm but if you squeeze hard, the stipe will shatter. Matsutake The stipe of the Matsutake is much denser and will not shatter (unless it is riddled with insect larvae and is no longer in good edible condition). There are other important differences. The flesh of Matsutake peels or shreds like string cheese. Also, the stipe of the Matsutake is widest near the gills Matsutake and tapers gradually to a point while the stipe of Amanita smithiana tends to be bulbous and is usually widest right at ground level. The partial veil and ring of a Matsutake is membranous while the partial veil and ring of Amanita smithiana is powdery and readily flocculates into small pieces (often disappearing entirely). For most people the difference in odor is very distinctive. Most collections of Amanita smithiana have a bleach-like odor while Matsutake has a distinctive smell of old gym socks and cinnamon redhots (however, not all people can distinguish the odors). -

The Current Status of the Family Russulaceae in the Uttarakhand Himalaya, India
Mycosphere Doi 10.5943/mycosphere/3/4/12 The current status of the family Russulaceae in the Uttarakhand Himalaya, India Joshi S1*, Bhatt RP1, and Stephenson SL2 1Departmet of Botany and Microbiology, H. N. B. Garhwal University, Srinagar Garhwal, Uttarakhand 246 174, India – [email protected], [email protected] 2Department of Biological Sciences, University of Arkansas, Fayetteville, Arkansas 72701, USA – [email protected] Joshi S, Bhatt RP, Stephenson SL 2012 – The current status of the family Russulaceae in the Uttarakhand Himalaya, India. Mycosphere 3(4), 486–501, Doi 10.5943 /mycosphere/3/4/12 The checklist provided herein represents a current assessment of what is known about the ectomycorrizal family Russulaceae from the Uttarakhand Himalaya. The checklist includes 105 taxa, 55 of which belong to the genus Lactarius Pers. ex S.F. Gray and 50 are members of the genus Russula Pers. ex S.F. Gray. Eleven of the species of Lactarius (listed as Lactarius sp. 1 to 11 in the checklist) are apparently new to science and have yet to be formally described. Key words – Ectomycorrhizal fungi – Himalayan Mountains – Lactarius – Russula – Taxonomy Article Information Received 24 July 2012 Accepted 31 July 2012 Published online 28 August 2012 *Corresponding author: Sweta Joshi – e-mail – [email protected] Introduction crucial niche relationships of the various The family Russulaceae is one of the elements that make up the forest biota, largest ectomycorrhizal families in the order including the macrofungi themselves. The Agaricales. The family was established by large gap that exists with respect to our Roze (1876) as the Russulariees (non. -

Bulletin of the Puget Sound Mycological Society No
BULLETIN OF THE PUGET SOUND MYCOLOGICAL SOCIETY Number 416 November 2005 MONSTER MOLD THREATENS HEALTH IN THE Clothes can be washed or dry cleaned, but most furniture is a SOUTH Julia Silverman and Marilynn Marchione loss. Ditto for carpeting, insulation, wallpaper, and drywall, which The Sporeprint, L.A. Myco. Soc., October 2005 no longer lives up to its name. Mattresses that didn’t get wet prob- ably have mold if they were in a room that did. “Anything with a New Orleans (AP) - Wearing goggles, gloves, ga- cushion you can forget about,” May said. loshes, and a mask, Veronica Randazzo lasted only The general advice is the same as when food is suspected of be- 10 minutes inside her home in St. Bernard Parish. Her eyes burned, her mouth filled with a salty taste, ing spoiled—when in doubt, throw it out. and she felt nauseous. Her 26-year-old daughter, When is professional help needed? “It’s simply a matter of ex- Alicia, also covered in gear, came out coughing. “That mold,” tent. If you’ve got small areas of mold, just a few square feet, it’s she said. “It smells like death.” something a homeowner can clean with 10 percent bleach,” said Anu Dixit, a fungus expert at Saint Louis University. Standing water after Hurricane Katrina created ideal growth condi- tions for mold and allowed it to penetrate so deep that experts fear Dixit studied mold after the Mississippi River floods in 1993 and that even studs of many homes are saturated and unsalvageable. 1994, and found cleaning measures often were ineffective, mainly Mold now forms an interior version of kudzu in the soggy South, because people started rebuilding too soon, before the surround- posing health dangers that will make many homes tear-downs and ing area was completely dry. -

Complete References List
Aanen, D. K. & T. W. Kuyper (1999). Intercompatibility tests in the Hebeloma crustuliniforme complex in northwestern Europe. Mycologia 91: 783-795. Aanen, D. K., T. W. Kuyper, T. Boekhout & R. F. Hoekstra (2000). Phylogenetic relationships in the genus Hebeloma based on ITS1 and 2 sequences, with special emphasis on the Hebeloma crustuliniforme complex. Mycologia 92: 269-281. Aanen, D. K. & T. W. Kuyper (2004). A comparison of the application of a biological and phenetic species concept in the Hebeloma crustuliniforme complex within a phylogenetic framework. Persoonia 18: 285-316. Abbott, S. O. & Currah, R. S. (1997). The Helvellaceae: Systematic revision and occurrence in northern and northwestern North America. Mycotaxon 62: 1-125. Abesha, E., G. Caetano-Anollés & K. Høiland (2003). Population genetics and spatial structure of the fairy ring fungus Marasmius oreades in a Norwegian sand dune ecosystem. Mycologia 95: 1021-1031. Abraham, S. P. & A. R. Loeblich III (1995). Gymnopilus palmicola a lignicolous Basidiomycete, growing on the adventitious roots of the palm sabal palmetto in Texas. Principes 39: 84-88. Abrar, S., S. Swapna & M. Krishnappa (2012). Development and morphology of Lysurus cruciatus--an addition to the Indian mycobiota. Mycotaxon 122: 217-282. Accioly, T., R. H. S. F. Cruz, N. M. Assis, N. K. Ishikawa, K. Hosaka, M. P. Martín & I. G. Baseia (2018). Amazonian bird's nest fungi (Basidiomycota): Current knowledge and novelties on Cyathus species. Mycoscience 59: 331-342. Acharya, K., P. Pradhan, N. Chakraborty, A. K. Dutta, S. Saha, S. Sarkar & S. Giri (2010). Two species of Lysurus Fr.: addition to the macrofungi of West Bengal. -
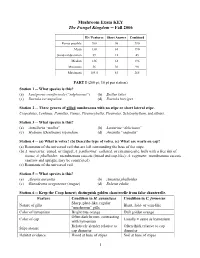
Mushroom Exam KEY the Fungal Kingdom -- Fall 2006
Mushroom Exam KEY The Fungal Kingdom -- Fall 2006 ID / Features Short Answer Combined Points possible 200 90 290 Mean 130 61 190 Standard deviation 39 12 45 Median 136 61 196 Minimum 36 36 90 Maximum 184.5 83 265 PART I (200 pt; 10 pt per station) Station 1 -- What species is this? (a) Laetiporus conifericola (“sulphureus”) (b) Suillus lakei (c) Russula xerampelina (d) Russula brevipes Station 2 -- Three genera of gilled mushrooms with no stipe or short lateral stipe. Crepidotus, Lentinus, Panellus, Panus, Pleurocybella, Pleurotus, Schizophyllum, and others. Station 3 -- What species is this? (a) Armillaria “mellea” (b) Lactarius “deliciosus” (c) Hydnum (Dentinum) repandum (d) Amanita “vaginata” Station 4 -- (a) What is volva? (b) Describe type of volva. (c) What are warts on cap? (a) Remnants of the universal veil that are left surrounding the base of the stipe. (b) A. muscaria: zoned, or ringed; A. pantherina: collared, or circumsessile, bulb with a free rim of tissue; A. phalloides: membranous saccate (broad and cup-like); A. vaginata: membranous saccate (narrow and upright, may be constricted) (c) Remnants of the universal veil. Station 5 -- What species is this? (a) Aleuria aurantia (b) Amanita phalloides (c) Ganoderma oregonense (tsugae) (d) Boletus edulis Station 6 -- Keep the Coop honest; distinguish golden chanterelle from false chanterelle. Feature Condition in H. aurantiaca Condition in C. formosus Sharp, plate-like, regular Nature of gills Blunt, fold- or vein-like “mushroom” gills Color of hymenium Bright true orange Dull golden orange Often dark brown, contrasting Color of cap Usually ± same as hymenium with hymenium Relatively slender relative to Often thick relative to cap Stipe stature cap diameter diameter Habitat evidence Wood at base of stipes Soil at base of stipes 1 Feature Condition in H. -

Final Report
NATURAL RESOURCE INVENTORY of the WHITE OAK POND WATERSHED Ashland, Center Harbor, & Holderness, NH FINAL REPORT [White Oak Pond as seen from the northeast shoreline] Compiled by: Dr. Rick Van de Poll Ecosystem Management Consultants 30 N. Sandwich Rd. Center Sandwich, NH 03227 603‐284‐6851 [email protected] Submitted to: Squam Lakes Conservation Society December 2020 i ii SUMMARY The 2982‐acre White Oak Pond watershed lies at the head of Mill Brook along Route 3 in Holderness, New Hampshire. It includes the 298‐acre, 35‐foot deep White Oak Pond (and islands) and its two primary drainage systems in Holderness, Ashland, and Center Harbor. The watershed forms the western part of the 28,094‐acre Squam Lake Drainage (HUC 010700010502) and lies immediately above Piper Cove on Squam Lake. The two perennial streams total 2.26 miles, with the largest one rising on the north slopes of McCrillis Hill in Center Harbor and flowing northerly, and the slightly smaller one draining an unnamed hill in the eastern corner of Ashland and flowing easterly through a large beaver marsh on Coxboro Road. The watershed is primarily forested, although ponds, wetlands and other surface waters make up a substantial portion of the area (23.8%). Forests are primarily mixed hardwoods and conifers, with an abundance of white pine and red oak that have regenerated from former pastureland. Forested wetlands make up the plurality of the hydric soils areas, where red maple swamps are the most common. Other commercially viable timber species include red spruce, eastern hemlock, sugar maple, yellow birch, beech, and white oak.