S FINCHES: <I>GEOSPIZA CONIROSTRIS</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

01 Grant 1-12.Qxd
COPYRIGHT NOTICE: Peter R. Grant & B. Rosemary Grant: How and Why Species Multiply is published by Princeton University Press and copyrighted, © 2007, by Princeton University Press. All rights reserved. No part of this book may be reproduced in any form by any electronic or mechanical means (including photocopying, recording, or information storage and retrieval) without permission in writing from the publisher, except for reading and browsing via the World Wide Web. Users are not permitted to mount this file on any network servers. Follow links for Class Use and other Permissions. For more information send email to: [email protected] CHAPTER ONE The Biodiversity Problem and Darwin’s Finches Now it is a well-known principle of zoological evolution that an isolated region, if large and sufficiently varied in topography, soil, climate and vegetation, will give rise to a diversified fauna according to the law of adaptive radiation from primitive and central types. Branches will spring off in all directions to take advantage of every possible opportunity of securing foods. (Osborn 1900, p. 563) I have stated that in the thirteen species of ground-finches, a nearly perfect gradation may be traced, from a beak extraordinarily thick, to one so fine, that it may be compared to that of a warbler. (Darwin 1839, p. 475) Biodiversity e live in a world so rich in species we do not know how many there are. Adding up every one we know, from influenza viruses Wto elephants, we reach a total of a million and a half (Wilson 1992, ch. 8). The real number is almost certainly at least five million, perhaps ten or even twenty, and although very large it is a small fraction of those that have ever existed; the vast majority has become extinct. -

Bio 209 Course Title: Chordates
BIO 209 CHORDATES NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 209 COURSE TITLE: CHORDATES 136 BIO 209 MODULE 4 MAIN COURSE CONTENTS PAGE MODULE 1 INTRODUCTION TO CHORDATES…. 1 Unit 1 General Characteristics of Chordates………… 1 Unit 2 Classification of Chordates…………………... 6 Unit 3 Hemichordata………………………………… 12 Unit 4 Urochordata………………………………….. 18 Unit 5 Cephalochordata……………………………... 26 MODULE 2 VERTEBRATE CHORDATES (I)……... 31 Unit 1 Vertebrata…………………………………….. 31 Unit 2 Gnathostomata……………………………….. 39 Unit 3 Amphibia…………………………………….. 45 Unit 4 Reptilia……………………………………….. 53 Unit 5 Aves (I)………………………………………. 66 Unit 6 Aves (II)……………………………………… 76 MODULE 3 VERTEBRATE CHORDATES (II)……. 90 Unit 1 Mammalia……………………………………. 90 Unit 2 Eutherians: Proboscidea, Sirenia, Carnivora… 100 Unit 3 Eutherians: Edentata, Artiodactyla, Cetacea… 108 Unit 4 Eutherians: Perissodactyla, Chiroptera, Insectivora…………………………………… 116 Unit 5 Eutherians: Rodentia, Lagomorpha, Primata… 124 MODULE 4 EVOLUTION, ADAPTIVE RADIATION AND ZOOGEOGRAPHY………………. 136 Unit 1 Evolution of Chordates……………………… 136 Unit 2 Adaptive Radiation of Chordates……………. 144 Unit 3 Zoogeography of the Nearctic and Neotropical Regions………………………………………. 149 Unit 4 Zoogeography of the Palaearctic and Afrotropical Regions………………………………………. 155 Unit 5 Zoogeography of the Oriental and Australasian Regions………………………………………. 160 137 BIO 209 CHORDATES COURSE GUIDE BIO 209 CHORDATES Course Team Prof. Ishaya H. Nock (Course Developer/Writer) - ABU, Zaria Prof. T. O. L. Aken’Ova (Course -

Inbreeding in Darwin's Medium Ground Finches (Geospiza Fortis)
Evohaion, 43(6),1989, pp. 1273-1284 INBREEDING IN DARWIN'S MEDIUM GROUND FINCHES (GEOSPIZA FORTIS) H, LISLE GIBBS'" Museum ofZoology and Department ofBiology, The University ofMichigan, Ann Arbor, MI48109-1079 AND PETER R. GRANT Department ofBiology, Princeton University, Princeton, NJ 08544-1008 Abstract. - We studied the frequency and causes of inbreeding and its effect on reproductive success in a population of Darwin's Medium Ground Finches iGeospiza fortis) on Isla Daphne Major, Galapagos, during four breeding seasons (1981, 1983, 1984, and 1987). Pedigree analysis showed that levels of inbreeding were low but comparable with those observed in other passerine birds. For pairs with at least half of their grandparents known, approximately 20% of all pairings were between detectably related birds. The frequency ofpairings between closely related birds (coefficient of kinship [</>] ~ 0.250) among all pairs was 0.6%. We detected no effect of inbreeding on repro ductive success, although sample sizes were small. The observed reproductive output of related pairs was not significantly different from the output ofunrelated pairs, and there was no correlation between a pair's kinship coefficient and an estimate of the potential magnitude of inbreeding depression. Comparisons with a study ofGreat Tits (Parus major) by van Noordwijk and Scharloo (1981) suggest that, even if present, the fitness costs of inbreeding in this population of G. fortis would be low. Observed levels of inbreeding in each breeding episode were accurately predicted by simulations of random mating in which relatedness had no influence on pairing between in dividuals. This result suggests that levels ofinbreeding in this population are determined more by demographic factors than by behavioral avoidance of mating with kin. -
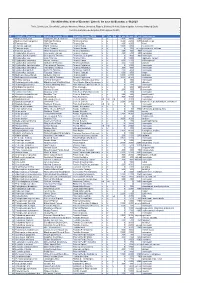
Ecuador Simple List Version 2020 Clements
Checklist of the birds of Ecuador / Lista de las aves del Ecuador, v. 08.2020 Freile, Brinkhuizen, Greenfield, Lysinger, Navarrete, Nilsson, Olmstead, Ridgely, Sánchez-Nivicela, Solano-Ugalde, Athanas, Ahlman & Boyla Comité Ecuatoriano de Registros Ornitológicos (CERO) ID Scientific_Clements_2019 English_Clements_2019 Español Ecuador CERO EC Con Gal Alt_min Alt_max Alt_ext Subespecies 1 Nothocercus julius Tawny-breasted Tinamou Tinamú Pechileonado x x 2300 3400 2100 monotypic 2 Nothocercus bonapartei Highland Tinamou Tinamú Serrano x x 1600 2200 3075 plumbeiceps 3 Tinamus tao Gray Tinamou Tinamú Gris x x 400 1600 kleei 4 Tinamus osgoodi Black Tinamou Tinamú Negro x x 1000 1400 hershkovitzi 5 Tinamus major Great Tinamou Tinamú Grande x x 0 700 1200, 1350 peruvianus, latifrons 6 Tinamus guttatus White-throated Tinamou Tinamú Goliblanco x x 200 400 900 monotypic 7 Crypturellus cinereus Cinereous Tinamou Tinamú Cinéreo x x 200 600 900 monotypic 8 Crypturellus berlepschi Berlepsch's Tinamou Tinamú de Berlepsch x x 0 400 900 monotypic 9 Crypturellus soui Little Tinamou Tinamú Chico x x 0 1200 nigriceps, harterti 10 Crypturellus obsoletus Brown Tinamou Tinamú Pardo x x 500 1100 chirimotanus? 11 Crypturellus undulatus Undulated Tinamou Tinamú Ondulado x x 200 600 yapura 12 Crypturellus transfasciatus Pale-browed Tinamou Tinamú Cejiblanco x x 0 1600 monotypic 13 Crypturellus variegatus Variegated Tinamou Tinamú Abigarrado x x 200 400 monotypic 14 Crypturellus bartletti Bartlett's Tinamou Tinamú de Bartlett x x 200 400 monotypic 15 Crypturellus -

Order Evolutionary Adaptation Response to the Environment
Order Response to the environment Evolutionary adaptation Reproduction Regulation Energy processing Growth and development 1 The biosphere Tissues Ecosystems Organs and organ systems Communities Cells Organelles Organisms Atoms Populations Molecules 2 Sunlight Leaves absorb light energy from Leaves take in the sun. carbon dioxide CO2 from the air and release oxygen. O2 Cycling of chemical nutrients Leaves fall to Water and Animals eat the ground and minerals in leaves and fruit are decomposed the soil are from the tree. by organisms taken up by that return the tree minerals to the through soil. its roots. 3 Sunlight Heat When energy is used Producers absorb light to do work, some energy and transform it into energy is converted to chemical energy. thermal energy, which is lost as heat. An animal’s muscle cells convert Chemical chemical energy energy from food to kinetic energy, the energy of motion. A plant’s cells use chemical energy to do Chemical energy in work such as growing food is transferred new leaves. from plants to consumers. (a) Energy flow from sunlight to (b) Using energy to do work producers to consumers 4 (a) Wings (b) Wing bones 5 Prokaryotic cell Eukaryotic cell DNA (no nucleus) Membrane Membrane Cytoplasm Nucleus (membrane- enclosed) Membrane- DNA (throughout enclosed organelles nucleus) 1 µm 6 Sperm cell Nuclei containing DNA Fertilized egg with DNA from Embryo’s cells with Egg cell both parents copies of inherited DNA Offspring with traits inherited from both parents 7 Nucleus A DNA C Nucleotide T A T Cell A C C G T A G T A (a) DNA double helix (b) Single strand of DNA 8 A Negative feedback Enzyme 1 B D Enzyme 2 Excess D blocks a step. -

Fiftee N Vertebrate Beginnings the Chordates
Hickman−Roberts−Larson: 15. Vertebrate Beginnings: Text © The McGraw−Hill Animal Diversity, Third The Chordates Companies, 2002 Edition 15 chapter •••••• fifteen Vertebrate Beginnings The Chordates It’s a Long Way from Amphioxus Along the more southern coasts of North America, half buried in sand on the seafloor,lives a small fishlike translucent animal quietly filtering organic particles from seawater.Inconspicuous, of no commercial value and largely unknown, this creature is nonetheless one of the famous animals of classical zoology.It is amphioxus, an animal that wonderfully exhibits the four distinctive hallmarks of the phylum Chordata—(1) dorsal, tubular nerve cord overlying (2) a supportive notochord, (3) pharyngeal slits for filter feeding, and (4) a postanal tail for propulsion—all wrapped up in one creature with textbook simplicity. Amphioxus is an animal that might have been designed by a zoologist for the classroom. During the nineteenth century,with inter- est in vertebrate ancestry running high, amphioxus was considered by many to resemble closely the direct ancestor of the vertebrates. Its exalted position was later acknowledged by Philip Pope in a poem sung to the tune of “Tipperary.”It ends with the refrain: It’s a long way from amphioxus It’s a long way to us, It’s a long way from amphioxus To the meanest human cuss. Well,it’s good-bye to fins and gill slits And it’s welcome lungs and hair, It’s a long, long way from amphioxus But we all came from there. But amphioxus’place in the sun was not to endure.For one thing,amphioxus lacks one of the most important of vertebrate charac- teristics,a distinct head with special sense organs and the equipment for shifting to an active predatory mode of life. -

Proceedings of the General Meetings for Scientific Business of The
THIS BOOK VM HOT BE PHOTOCOPIED -—'•»r.-»«a!! 190 Page Page {94 Zeus roseus 1843, 85 Ifift Zonites fuliginosus .... 1834, 63 walkeri 1834, 63 Zanclus comutus 1833, 117 Zoothera 1830-1, 172 Zapomia pusilla 1839, 134 u.onticola • • • • Zebiida adamsii 1847, 121 j ^^S; ^^ rj ., • 11843, 115 Zonotrichia matutina . 1843, 113 Zenaida am-ita nSJ.? ^'\ Zophosis nodosa 1841, 116 Zeus aper 1833,' 114 Zosterops 1845, 24 childreni 1843, 85 albogularis 1836, 75 concMfer 1845, 103 chloronotus 1840, 165 1839, 82 maderaspatanus .... 1839, 161 f*^'foi^, •••• ) 11846; 27 tenuirostris 1836, 76 --% THE END OF THE INDEX. PROCEEDINGS ZOOLOGICAL SOCIETY OF LONDON. INDEX. 1848—1860. PRINTED FOE THE SOCIETY; SOLD AT THEIE HOUSE IN HANOVEE SQUARE AND AT MESSRS. LONGaiAN. GREEN, LONGMANS, AND ROBERTS. PATEENOSTER-ROW. 1863. PRINTED BY TATXOR AND FRANCIS, BED LION COURT, FLEET STKEET. CONTENTS. Page List of the Names of Contributors, from 1848 to 1860, with the Titles of and References to the several Articles con- tributed by each 1 List of the Illustrations, 1848 to 1860 67 Index of Species described and referred to, 1848 to 1860 .... 91 LIST OF THE NAMES 08" CONTRIBUTORS, From 1848 to 1860, With the Titles of and References to the several Articles contributed by each. Page Adams, A. Leith, M.U., A.M., Surgeon 22Qd Regiment. Notes on the Habits, Haunts, &c. of some of the Birds of India (communicated by Messrs. T. J. and F. Moore) 1858, 466 Remarks on the Habits and Haunts of some of the Mammalia found in various parts of India and the West Himalayan Mountains (communicated by Messrs. -

Bird Checklists of the World Country Or Region
Avibase Page 1of 44 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Ecuador 2 Number of species: 1665 3 Number of endemics: 32 4 Number of breeding endemics: 2 5 Number of globally threatened species: 103 6 Number of extinct species: 0 7 Number of introduced species: 6 8 Date last reviewed: 2016-07-31 9 10 Recommended citation: Lepage, D. 2016. Checklist of the birds of Ecuador. Avibase, the world bird database. Retrieved from http://avibase.bsc- eoc.org/checklist.jsp?lang=EN®ion=ec&list=howardmoore&format=1 on 10/10/2016. Make your observations count! Submit your data to ebird. -

Genetic Adaptation and Speciation in Darwin S Finches and Atlantic Herring
!"#!$# % & ' &&%%& ($!"## ! " # $# $$ %%& ' " & & (' ')# & *+,' - . "'+# ( & /01" 2 3 ) & . " . 1 ')..1* & &4 5 - *+ ! #+$$+6 7 1 - 8# ' 7 ! "+ %9$+:; + 7 +<15;=;%%$9+ 5 "" " & " " '+< - ' "' ' ' > ' ' " & -''' ? & '& & +,'' - - 8& ' 7 ' " '" ' " " + ,' " & - 8 & ' ' 6 @ " < & 3 ' "+ "-' " ? " & - 8 & ' - ' '"' -' 3>+ & & "'' -' ' & " ' "" & ' " & '! + 6 & " & " " & " -' "'" & +. ' &'" & " 3" +< " "' ' "' ' - - +A ' &" - 8& ' & ' ' " 3 '-''" 3 ' "+ ! > " " > -'' +A - &' > - 8& '+< '& - " & - 8& '- & ' "'' > - " & '! " . B & '! +< ' & " & " & '! & '!+4" ' " &' & '' -' " ' &' > + A" ' &'7 ' "-' > &=$9 -'' -' '"' 3 " C + ,'& ' & & D -''3 -' " ' " && - + ,' " & ' & ' - & '"' & " & &'' "" + "# $ - E& '7 ' "( " . . " 1 % & ' %& ()*% %+,)-. %#! F# ! $$ <115%9%9$9 <15;=;%%$9 ;=9)' GG +3+G H I ;=9* To the future List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Lamichhaney S, Han F, Berglund J, Wang C, Sällman Almen, M, Webster MT, Grant BR, Grant PR, Andersson L. (2016). A beak size locus -

Lamichhaney Et Al. 2015. Evolution of Darwin's Finches and Their Beaks
ARTICLE doi:10.1038/nature14181 Evolution of Darwin’s finches and their beaks revealed by genome sequencing Sangeet Lamichhaney1*, Jonas Berglund1*, Markus Sa¨llman Alme´n1, Khurram Maqbool2, Manfred Grabherr1, Alvaro Martinez-Barrio1, Marta Promerova´1, Carl-Johan Rubin1, Chao Wang1, Neda Zamani1,3, B. Rosemary Grant4, Peter R. Grant4, Matthew T. Webster1 & Leif Andersson1,2,5 Darwin’s finches, inhabiting the Gala´pagos archipelago and Cocos Island, constitute an iconic model for studies of speci- ation and adaptive evolution. Here we report the results of whole-genome re-sequencing of 120 individuals representing all of the Darwin’s finch species and two close relatives. Phylogenetic analysis reveals important discrepancies with the phenotype-based taxonomy. We find extensive evidence for interspecific gene flow throughout the radiation. Hybrid- ization has given rise to species of mixed ancestry. A 240 kilobase haplotype encompassing the ALX1 gene that encodes a transcription factor affecting craniofacial development is strongly associated with beak shape diversity across Darwin’s finch species as well as within the medium ground finch (Geospiza fortis), a species that has undergone rapid evolution of beak shape in response to environmental changes. The ALX1 haplotype has contributed to diversification of beak shapes among the Darwin’s finches and, thereby, to an expanded utilization of food resources. Adaptive radiations are particularly informative for understanding the diversification throughout phylogeny, and report the discovery of a locus ecological and genetic basis of biodiversity1,2. Those causes are best iden- with a major effect on beak shape. tified in young radiations, as they represent the early stages of diver- sification when phenotypic transitions between species are small and Considerable nucleotide diversity interpretable and extinctions are likely to be minimal3. -

Evolution of Ecological Diversity in the Neotropical Tanagers of the Genus
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2003 Evolution of ecological diversity in the neotropical tanagers of the genus Tangara (Aves: Thraupidae) Kazuya Naoki Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Recommended Citation Naoki, Kazuya, "Evolution of ecological diversity in the neotropical tanagers of the genus Tangara (Aves: Thraupidae)" (2003). LSU Doctoral Dissertations. 2021. https://digitalcommons.lsu.edu/gradschool_dissertations/2021 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. EVOLUTION OF ECOLOGICAL DIVERSITY IN THE NEOTROPICAL TANAGERS OF THE GENUS TANGARA (AVES: THRAUPIDAE) A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Biological Sciences by Kazuya Naoki B.S., Universidad de Costa Rica, 1996 August 2003 ACKNOWLEDGMENTS Many people and institutions in many countries helped make this dissertation possible. First I thank my parents and my sister in Japan to understand and support my interests in tropical biology for last 14 years. Even though I have failed to get in touch with them frequently, they always showed great comprehension on my passion and love to nature and offered to support me in all possible ways. I thank my advisor, J. -

Diversity and Movement Patterns of Passerine Birds Near an Urban Center on Santa Cruz, Galapagos Islands Ana M
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 January 2007 Diversity And Movement Patterns Of Passerine Birds Near An Urban Center On Santa Cruz, Galapagos Islands Ana M. Gabela University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/theses Gabela, Ana M., "Diversity And Movement Patterns Of Passerine Birds Near An Urban Center On Santa Cruz, Galapagos Islands" (2007). Masters Theses 1911 - February 2014. 20. Retrieved from https://scholarworks.umass.edu/theses/20 This thesis is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses 1911 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. DIVERSITY AND MOVEMENT PATTERNS OF PASSERINE BIRDS NEAR AN URBAN CENTER ON SANTA CRUZ, GALAPAGOS ISLANDS A Thesis Presented By ANA MARIA GABELA Submitted to the Graduate School of the University of Massachussetss Amherst in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE May 2007 Graduate Program in Organismic and Evolutionary Biology © Copyright by Ana Maria Gabela 2007 All Rights Reserved DIVERSITY AND MOVEMENT PATTERNS OF PASSERINE BIRDS NEAR AN URBAN CENTER ON SANTA CRUZ, GALAPAGOS ISLANDS A Thesis Presented by ANA MARIA GABELA Approved as to style and content by: ____________________________________ Jeffrey E. Podos, Chair ____________________________________