Inbreeding in Darwin's Medium Ground Finches (Geospiza Fortis)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecuador & the Galapagos Islands
Ecuador & the Galapagos Islands - including Sacha Lodge Extension Naturetrek Tour Report 29 January – 20 February 2018 Medium Ground-finch Blue-footed Booby Wire-tailed Manakin Galapagos Penguin Green Sea Turtle Report kindly compiled by Tour participants Sally Wearing, Rowena Tye, Debbie Hardie and Sue Swift Images courtesy of David Griffiths, Sue Swift, Debbie Hardie, Jenny Tynan, Rowena Tye, Nick Blake and Sally Wearing Naturetrek Mingledown Barn Wolf’s Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Ecuador & the Galapagos Islands - including Sacha Lodge Extension Tour Leader in the Galapagos: Juan Tapia with 13 Naturetrek Clients This report has kindly been compiled by tour participants Sally Wearing, Rowena Tye, Debbie Hardie and Sue Swift. Day 1 Monday 29th January UK to Quito People arrived in Quito via Amsterdam with KLM or via Madrid with Iberia, while Tony came separately from the USA. Everyone was met at the airport and taken to the Hotel Vieja Cuba; those who were awake enough went out to eat before a good night’s rest. Day 2 Tuesday 30th January Quito. Weather: Hot and mostly sunny. The early risers saw the first few birds of the trip outside the hotel: Rufous- collared Sparrow, Great Thrush and Eared Doves. After breakfast, an excellent guide took us on a bus and walking tour of Quito’s old town. This started with the Basilica del Voto Nacional, where everyone marvelled at the “grotesques” of native Ecuadorian animals such as frigatebirds, iguanas and tortoises. -

01 Grant 1-12.Qxd
COPYRIGHT NOTICE: Peter R. Grant & B. Rosemary Grant: How and Why Species Multiply is published by Princeton University Press and copyrighted, © 2007, by Princeton University Press. All rights reserved. No part of this book may be reproduced in any form by any electronic or mechanical means (including photocopying, recording, or information storage and retrieval) without permission in writing from the publisher, except for reading and browsing via the World Wide Web. Users are not permitted to mount this file on any network servers. Follow links for Class Use and other Permissions. For more information send email to: [email protected] CHAPTER ONE The Biodiversity Problem and Darwin’s Finches Now it is a well-known principle of zoological evolution that an isolated region, if large and sufficiently varied in topography, soil, climate and vegetation, will give rise to a diversified fauna according to the law of adaptive radiation from primitive and central types. Branches will spring off in all directions to take advantage of every possible opportunity of securing foods. (Osborn 1900, p. 563) I have stated that in the thirteen species of ground-finches, a nearly perfect gradation may be traced, from a beak extraordinarily thick, to one so fine, that it may be compared to that of a warbler. (Darwin 1839, p. 475) Biodiversity e live in a world so rich in species we do not know how many there are. Adding up every one we know, from influenza viruses Wto elephants, we reach a total of a million and a half (Wilson 1992, ch. 8). The real number is almost certainly at least five million, perhaps ten or even twenty, and although very large it is a small fraction of those that have ever existed; the vast majority has become extinct. -

Can Darwin's Finches and Their Native Ectoparasites Survive the Control of Th
Insect Conservation and Diversity (2017) 10, 193–199 doi: 10.1111/icad.12219 FORUM & POLICY Coextinction dilemma in the Galapagos Islands: Can Darwin’s finches and their native ectoparasites survive the control of the introduced fly Philornis downsi? 1 2 MARIANA BULGARELLA and RICARDO L. PALMA 1School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand and 2Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand Abstract. 1. The survival of parasites is threatened directly by environmental alter- ation and indirectly by all the threats acting upon their hosts, facing coextinction. 2. The fate of Darwin’s finches and their native ectoparasites in the Galapagos Islands is uncertain because of an introduced avian parasitic fly, Philornis downsi, which could potentially drive them to extinction. 3. We documented all known native ectoparasites of Darwin’s finches. Thir- teen species have been found: nine feather mites, three feather lice and one nest mite. No ticks or fleas have been recorded from them yet. 4. Management options being considered to control P. downsi include the use of the insecticide permethrin in bird nests which would not only kill the invasive fly larvae but the birds’ native ectoparasites too. 5. Parasites should be targeted for conservation in a manner equal to that of their hosts. We recommend steps to consider if permethrin-treated cotton sta- tions are to be deployed in the Galapagos archipelago to manage P. downsi. Key words. Chewing lice, coextinction, Darwin’s finches, dilemma, ectoparasites, feather mites, Galapagos Islands, permethrin, Philornis downsi. Introduction species have closely associated species which are also endangered (Dunn et al., 2009). -

Bio 209 Course Title: Chordates
BIO 209 CHORDATES NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 209 COURSE TITLE: CHORDATES 136 BIO 209 MODULE 4 MAIN COURSE CONTENTS PAGE MODULE 1 INTRODUCTION TO CHORDATES…. 1 Unit 1 General Characteristics of Chordates………… 1 Unit 2 Classification of Chordates…………………... 6 Unit 3 Hemichordata………………………………… 12 Unit 4 Urochordata………………………………….. 18 Unit 5 Cephalochordata……………………………... 26 MODULE 2 VERTEBRATE CHORDATES (I)……... 31 Unit 1 Vertebrata…………………………………….. 31 Unit 2 Gnathostomata……………………………….. 39 Unit 3 Amphibia…………………………………….. 45 Unit 4 Reptilia……………………………………….. 53 Unit 5 Aves (I)………………………………………. 66 Unit 6 Aves (II)……………………………………… 76 MODULE 3 VERTEBRATE CHORDATES (II)……. 90 Unit 1 Mammalia……………………………………. 90 Unit 2 Eutherians: Proboscidea, Sirenia, Carnivora… 100 Unit 3 Eutherians: Edentata, Artiodactyla, Cetacea… 108 Unit 4 Eutherians: Perissodactyla, Chiroptera, Insectivora…………………………………… 116 Unit 5 Eutherians: Rodentia, Lagomorpha, Primata… 124 MODULE 4 EVOLUTION, ADAPTIVE RADIATION AND ZOOGEOGRAPHY………………. 136 Unit 1 Evolution of Chordates……………………… 136 Unit 2 Adaptive Radiation of Chordates……………. 144 Unit 3 Zoogeography of the Nearctic and Neotropical Regions………………………………………. 149 Unit 4 Zoogeography of the Palaearctic and Afrotropical Regions………………………………………. 155 Unit 5 Zoogeography of the Oriental and Australasian Regions………………………………………. 160 137 BIO 209 CHORDATES COURSE GUIDE BIO 209 CHORDATES Course Team Prof. Ishaya H. Nock (Course Developer/Writer) - ABU, Zaria Prof. T. O. L. Aken’Ova (Course -

The Size of Beaks of Darwin's Finches on the Galápagos Islands Is Influenced by Natural Selection
NOT FOR SALE TheT size of beaks of Darwin’s fi nches on the Galápagos 8 IIslands is infl uenced by natural selection. © Roberts and Company Publishers, ISBN: 9781936221172, due August 15, 2012, For examination purposes only FINAL PAGES ZE_Ch08_p218-253.indd 218 7/18/12 2:06 PM NOT FOR SALE Natural Selection Empirical Studies in the Wild Learning Objectives • Describe the diff erences between directional selection and stabilizing selection. • Demonstrate how predators can act as agents of selection. • Explain how scarlet kingsnakes can exhibit a colorful pattern in one part of their range and be much redder in another. • Defi ne extended phenotypes. • Explain how replicated natural experiments can be used to examine evolutionary change in response to selection. • Analyze how selective sweeps can be detected within genomes. • Describe three genetic changes that have been identifi ed in the evolution of maize. • Explain how Bt resistance came about in insects. • Explain how body size and gape width could have evolved in Australian snakes in response to the invasion of cane toads. • Discuss how fi shing regulations could aff ect growth rates of fi sh populations. Charles Darwin managed to visit only a handful of the Galápa- gos Islands in 1835 while on his journey aboard the Beagle. Among the many islands he passed by was a tiny volcanic cone known as Daphne Major. Even today, it is not an easy place to visit. To set foot on Daphne Major, you have to approach a steep cliff in a small boat and then take an acrobatic leap onto a tiny ledge. -
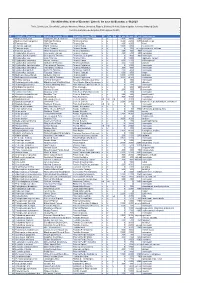
Ecuador Simple List Version 2020 Clements
Checklist of the birds of Ecuador / Lista de las aves del Ecuador, v. 08.2020 Freile, Brinkhuizen, Greenfield, Lysinger, Navarrete, Nilsson, Olmstead, Ridgely, Sánchez-Nivicela, Solano-Ugalde, Athanas, Ahlman & Boyla Comité Ecuatoriano de Registros Ornitológicos (CERO) ID Scientific_Clements_2019 English_Clements_2019 Español Ecuador CERO EC Con Gal Alt_min Alt_max Alt_ext Subespecies 1 Nothocercus julius Tawny-breasted Tinamou Tinamú Pechileonado x x 2300 3400 2100 monotypic 2 Nothocercus bonapartei Highland Tinamou Tinamú Serrano x x 1600 2200 3075 plumbeiceps 3 Tinamus tao Gray Tinamou Tinamú Gris x x 400 1600 kleei 4 Tinamus osgoodi Black Tinamou Tinamú Negro x x 1000 1400 hershkovitzi 5 Tinamus major Great Tinamou Tinamú Grande x x 0 700 1200, 1350 peruvianus, latifrons 6 Tinamus guttatus White-throated Tinamou Tinamú Goliblanco x x 200 400 900 monotypic 7 Crypturellus cinereus Cinereous Tinamou Tinamú Cinéreo x x 200 600 900 monotypic 8 Crypturellus berlepschi Berlepsch's Tinamou Tinamú de Berlepsch x x 0 400 900 monotypic 9 Crypturellus soui Little Tinamou Tinamú Chico x x 0 1200 nigriceps, harterti 10 Crypturellus obsoletus Brown Tinamou Tinamú Pardo x x 500 1100 chirimotanus? 11 Crypturellus undulatus Undulated Tinamou Tinamú Ondulado x x 200 600 yapura 12 Crypturellus transfasciatus Pale-browed Tinamou Tinamú Cejiblanco x x 0 1600 monotypic 13 Crypturellus variegatus Variegated Tinamou Tinamú Abigarrado x x 200 400 monotypic 14 Crypturellus bartletti Bartlett's Tinamou Tinamú de Bartlett x x 200 400 monotypic 15 Crypturellus -

S FINCHES: <I>GEOSPIZA CONIROSTRIS</I>
EVOLUTION INTERNATIONAL JOURNAL OF ORGANIC EVOLUTION PUBLISHED BY THE SOCIETY FOR THE STUDY OF EVOLUTION Vol. 36 July, 1982 No.4 Evolution, 36(4), 1982, pp. 637-657 NICHE SHIFTS AND COMPETITION IN DARWIN'S FINCHES: GEOSPIZA CONIROSTRIS AND CONGENERS B. R. GRANT AND P. R. GRANT Division of Biological Sciences, University of Michigan, Ann Arbor, Michigan 48109 Received February 5, 1981. Revised October 20, 1981 The idea that interspecific competition titative ecological data to the hypotheses, is an important process in structuring because quantitative data were not used communities stems largely from David to construct the hypotheses. In fact, Lack Lack's work with Darwin's Finches (Lack, had almost no ecological data (see Abbott 1940, 1945, 1947, 1969). Lack made eco et al., 1977). logical inferences about feeding niches In our initial studies we analyzed eco from analyses of bill sizes and shapes. He logical and morphological data from six listed several instances where the niches species on eight Galapagos islands (Abbott of coexisting species were different, and et al., 1977; Smith et al., 1978). To follow where the niche of an absent species ap this general approach, we selected for more peared to be occupied by one or more detailed study three situations which have species present. These examples he inter been heralded as especially clear illustra preted as evidence of competitive dis tions of competitive effects (e.g., Lack, placement and exclusion. 1969; Williamson, 1972; Arms and Camp, Lack's aim was to offer a coherent the 1979), the small size of Geospizafortis on oretical framework for understanding the Isla Daphne Major in the absence of G. -
![The Origin of Species: the Beak of the Finch [NARRATOR:] Our Planet](https://docslib.b-cdn.net/cover/9829/the-origin-of-species-the-beak-of-the-finch-narrator-our-planet-2889829.webp)
The Origin of Species: the Beak of the Finch [NARRATOR:] Our Planet
The Origin of Species: The Beak of the Finch [NARRATOR:] Our planet has millions of species. Over 300,000 beetles alone. 17,000 butterflies. Thousands of mammals, fish and birds, all astonishingly different. How did so many species come to be? To seek insights into that question, researchers are focusing on places where species recently arose, such as the remote Galápagos Islands. [CARROLL:] Scientists are making observations and conducting experiments that would have surprised Charles Darwin. And they're discovering new insights into what the great naturalist called the "mystery of mysteries": How new species form. [NARRATOR:] The Galápagos Islands are one of the most spectacular landscapes in the world, home to a variety of species that live nowhere else. Biologists Peter and Rosemary Grant have been seeking answers to how species arise by focusing on one of the smaller islands, called Daphne Major. [PETER GRANT:] When we started out, we had no plan for the long term. In fact, we thought it was just going to be just a few years, maybe two years. [NARRATOR:] Two years have turned into a 40-year odyssey. The Grants have returned every summer since 1973. [ROSEMARY GRANT:] Oh, there's a bird. [PETER GRANT:] Is that 306? [ROSEMARY GRANT:] Three oh metal six. [NARRATOR:] Here, they've made some of the most remarkable observations in the history of field research as they studied the famed Galápagos finches. The finches were first brought to scientists' attention by Charles Darwin, when his voyage around South America brought him to this cluster of islands 600 miles from mainland Ecuador. -

Order Evolutionary Adaptation Response to the Environment
Order Response to the environment Evolutionary adaptation Reproduction Regulation Energy processing Growth and development 1 The biosphere Tissues Ecosystems Organs and organ systems Communities Cells Organelles Organisms Atoms Populations Molecules 2 Sunlight Leaves absorb light energy from Leaves take in the sun. carbon dioxide CO2 from the air and release oxygen. O2 Cycling of chemical nutrients Leaves fall to Water and Animals eat the ground and minerals in leaves and fruit are decomposed the soil are from the tree. by organisms taken up by that return the tree minerals to the through soil. its roots. 3 Sunlight Heat When energy is used Producers absorb light to do work, some energy and transform it into energy is converted to chemical energy. thermal energy, which is lost as heat. An animal’s muscle cells convert Chemical chemical energy energy from food to kinetic energy, the energy of motion. A plant’s cells use chemical energy to do Chemical energy in work such as growing food is transferred new leaves. from plants to consumers. (a) Energy flow from sunlight to (b) Using energy to do work producers to consumers 4 (a) Wings (b) Wing bones 5 Prokaryotic cell Eukaryotic cell DNA (no nucleus) Membrane Membrane Cytoplasm Nucleus (membrane- enclosed) Membrane- DNA (throughout enclosed organelles nucleus) 1 µm 6 Sperm cell Nuclei containing DNA Fertilized egg with DNA from Embryo’s cells with Egg cell both parents copies of inherited DNA Offspring with traits inherited from both parents 7 Nucleus A DNA C Nucleotide T A T Cell A C C G T A G T A (a) DNA double helix (b) Single strand of DNA 8 A Negative feedback Enzyme 1 B D Enzyme 2 Excess D blocks a step. -

Fiftee N Vertebrate Beginnings the Chordates
Hickman−Roberts−Larson: 15. Vertebrate Beginnings: Text © The McGraw−Hill Animal Diversity, Third The Chordates Companies, 2002 Edition 15 chapter •••••• fifteen Vertebrate Beginnings The Chordates It’s a Long Way from Amphioxus Along the more southern coasts of North America, half buried in sand on the seafloor,lives a small fishlike translucent animal quietly filtering organic particles from seawater.Inconspicuous, of no commercial value and largely unknown, this creature is nonetheless one of the famous animals of classical zoology.It is amphioxus, an animal that wonderfully exhibits the four distinctive hallmarks of the phylum Chordata—(1) dorsal, tubular nerve cord overlying (2) a supportive notochord, (3) pharyngeal slits for filter feeding, and (4) a postanal tail for propulsion—all wrapped up in one creature with textbook simplicity. Amphioxus is an animal that might have been designed by a zoologist for the classroom. During the nineteenth century,with inter- est in vertebrate ancestry running high, amphioxus was considered by many to resemble closely the direct ancestor of the vertebrates. Its exalted position was later acknowledged by Philip Pope in a poem sung to the tune of “Tipperary.”It ends with the refrain: It’s a long way from amphioxus It’s a long way to us, It’s a long way from amphioxus To the meanest human cuss. Well,it’s good-bye to fins and gill slits And it’s welcome lungs and hair, It’s a long, long way from amphioxus But we all came from there. But amphioxus’place in the sun was not to endure.For one thing,amphioxus lacks one of the most important of vertebrate charac- teristics,a distinct head with special sense organs and the equipment for shifting to an active predatory mode of life. -

Proceedings of the General Meetings for Scientific Business of The
THIS BOOK VM HOT BE PHOTOCOPIED -—'•»r.-»«a!! 190 Page Page {94 Zeus roseus 1843, 85 Ifift Zonites fuliginosus .... 1834, 63 walkeri 1834, 63 Zanclus comutus 1833, 117 Zoothera 1830-1, 172 Zapomia pusilla 1839, 134 u.onticola • • • • Zebiida adamsii 1847, 121 j ^^S; ^^ rj ., • 11843, 115 Zonotrichia matutina . 1843, 113 Zenaida am-ita nSJ.? ^'\ Zophosis nodosa 1841, 116 Zeus aper 1833,' 114 Zosterops 1845, 24 childreni 1843, 85 albogularis 1836, 75 concMfer 1845, 103 chloronotus 1840, 165 1839, 82 maderaspatanus .... 1839, 161 f*^'foi^, •••• ) 11846; 27 tenuirostris 1836, 76 --% THE END OF THE INDEX. PROCEEDINGS ZOOLOGICAL SOCIETY OF LONDON. INDEX. 1848—1860. PRINTED FOE THE SOCIETY; SOLD AT THEIE HOUSE IN HANOVEE SQUARE AND AT MESSRS. LONGaiAN. GREEN, LONGMANS, AND ROBERTS. PATEENOSTER-ROW. 1863. PRINTED BY TATXOR AND FRANCIS, BED LION COURT, FLEET STKEET. CONTENTS. Page List of the Names of Contributors, from 1848 to 1860, with the Titles of and References to the several Articles con- tributed by each 1 List of the Illustrations, 1848 to 1860 67 Index of Species described and referred to, 1848 to 1860 .... 91 LIST OF THE NAMES 08" CONTRIBUTORS, From 1848 to 1860, With the Titles of and References to the several Articles contributed by each. Page Adams, A. Leith, M.U., A.M., Surgeon 22Qd Regiment. Notes on the Habits, Haunts, &c. of some of the Birds of India (communicated by Messrs. T. J. and F. Moore) 1858, 466 Remarks on the Habits and Haunts of some of the Mammalia found in various parts of India and the West Himalayan Mountains (communicated by Messrs. -

Bird Checklists of the World Country Or Region
Avibase Page 1of 44 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Ecuador 2 Number of species: 1665 3 Number of endemics: 32 4 Number of breeding endemics: 2 5 Number of globally threatened species: 103 6 Number of extinct species: 0 7 Number of introduced species: 6 8 Date last reviewed: 2016-07-31 9 10 Recommended citation: Lepage, D. 2016. Checklist of the birds of Ecuador. Avibase, the world bird database. Retrieved from http://avibase.bsc- eoc.org/checklist.jsp?lang=EN®ion=ec&list=howardmoore&format=1 on 10/10/2016. Make your observations count! Submit your data to ebird.