SEXUAL SELECTION on BODY SIZE, TERRITORY and PLUMAGE VARIABLES in a POPULATION of DARWIN&Apos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Structured Decision Making to Prioritize Species Assemblages for Conservation T ⁎ Adam W
Journal for Nature Conservation 45 (2018) 48–57 Contents lists available at ScienceDirect Journal for Nature Conservation journal homepage: www.elsevier.com/locate/jnc Using Structured Decision Making to prioritize species assemblages for conservation T ⁎ Adam W. Greena, , Maureen D. Corrella, T. Luke Georgea, Ian Davidsonb, Seth Gallagherc, Chris Westc, Annamarie Lopatab, Daniel Caseyd, Kevin Ellisone, David C. Pavlacky Jr.a, Laura Quattrinia, Allison E. Shawa, Erin H. Strassera, Tammy VerCauterena, Arvind O. Panjabia a Bird Conservancy of the Rockies, 230 Cherry St., Suite 150, Fort Collins, CO, 80521, USA b National Fish and Wildlife Foundation, 1133 15th St NW #1100, Washington, DC, 20005, USA c National Fish and Wildlife Foundation, 44 Cook St, Suite 100, Denver, CO, 80206, USA d Northern Great Plains Joint Venture, 3302 4th Ave. N, Billings, MT, 59101, USA e World Wildlife Fund, Northern Great Plains Program, 13 S. Willson Ave., Bozeman, MT, 59715, USA ARTICLE INFO ABSTRACT Keywords: Species prioritization efforts are a common strategy implemented to efficiently and effectively apply con- Conservation planning servation efforts and allocate resources to address global declines in biodiversity. These structured processes help Grasslands identify species that best represent the entire species community; however, these methods are often subjective Priority species and focus on a limited number of species characteristics. We developed an objective, transparent approach using Prioritization a Structured Decision Making (SDM) framework to identify a group of grassland bird species on which to focus Structured decision making conservation efforts that considers biological, social, and logistical criteria in the Northern Great Plains of North America. The process quantified these criteria to ensure representation of a variety of species and habitats and included the relative value of each criterion to the working group. -

01 Grant 1-12.Qxd
COPYRIGHT NOTICE: Peter R. Grant & B. Rosemary Grant: How and Why Species Multiply is published by Princeton University Press and copyrighted, © 2007, by Princeton University Press. All rights reserved. No part of this book may be reproduced in any form by any electronic or mechanical means (including photocopying, recording, or information storage and retrieval) without permission in writing from the publisher, except for reading and browsing via the World Wide Web. Users are not permitted to mount this file on any network servers. Follow links for Class Use and other Permissions. For more information send email to: [email protected] CHAPTER ONE The Biodiversity Problem and Darwin’s Finches Now it is a well-known principle of zoological evolution that an isolated region, if large and sufficiently varied in topography, soil, climate and vegetation, will give rise to a diversified fauna according to the law of adaptive radiation from primitive and central types. Branches will spring off in all directions to take advantage of every possible opportunity of securing foods. (Osborn 1900, p. 563) I have stated that in the thirteen species of ground-finches, a nearly perfect gradation may be traced, from a beak extraordinarily thick, to one so fine, that it may be compared to that of a warbler. (Darwin 1839, p. 475) Biodiversity e live in a world so rich in species we do not know how many there are. Adding up every one we know, from influenza viruses Wto elephants, we reach a total of a million and a half (Wilson 1992, ch. 8). The real number is almost certainly at least five million, perhaps ten or even twenty, and although very large it is a small fraction of those that have ever existed; the vast majority has become extinct. -

L O U I S I a N A
L O U I S I A N A SPARROWS L O U I S I A N A SPARROWS Written by Bill Fontenot and Richard DeMay Photography by Greg Lavaty and Richard DeMay Designed and Illustrated by Diane K. Baker What is a Sparrow? Generally, sparrows are characterized as New World sparrows belong to the bird small, gray or brown-streaked, conical-billed family Emberizidae. Here in North America, birds that live on or near the ground. The sparrows are divided into 13 genera, which also cryptic blend of gray, white, black, and brown includes the towhees (genus Pipilo), longspurs hues which comprise a typical sparrow’s color (genus Calcarius), juncos (genus Junco), and pattern is the result of tens of thousands of Lark Bunting (genus Calamospiza) – all of sparrow generations living in grassland and which are technically sparrows. Emberizidae is brushland habitats. The triangular or cone- a large family, containing well over 300 species shaped bills inherent to most all sparrow species are perfectly adapted for a life of granivory – of crushing and husking seeds. “Of Louisiana’s 33 recorded sparrows, Sparrows possess well-developed claws on their toes, the evolutionary result of so much time spent on the ground, scratching for seeds only seven species breed here...” through leaf litter and other duff. Additionally, worldwide, 50 of which occur in the United most species incorporate a substantial amount States on a regular basis, and 33 of which have of insect, spider, snail, and other invertebrate been recorded for Louisiana. food items into their diets, especially during Of Louisiana’s 33 recorded sparrows, Opposite page: Bachman Sparrow the spring and summer months. -

Sharing Your Land with Prairie Wildlife
Sharing Your Land with Prairie Wildlife Scott W. Gillihan, David J. Hanni, Scott W. Hutchings, Tony Leukering, Ted Toombs, and Tammy VerCauteren Rocky Mountain Bird Observatory Rocky Mountain Bird Observatory Rocky Mountain Bird Observatory Sharing Your Land with Prairie Wildlife Scott W. Gillihan, David J. Hanni, Scott W. Hutchings, Tony Leukering, Ted Toombs, and Tammy VerCauteren 14500 Lark Bunting Rocky Mountain Bird Observatory Lane Brighton, CO 80603 (303) 659-4348 www.rmbo.org AboutIntroduction the Rocky Mountain Bird Observatory (RMBO): Our mission is to conserve Rocky Mountain, Great Plains, and Intermountain West birds and their habitats through research, monitoring, education, and outreach. We conduct on-the-ground conservation in cooperation with other private organizations and government agencies responsible for managing areas and programs important for birds. We also work with private landowners and managers to encourage practices that foster good land stewardship. Much of our work is designed to increase understanding of birds and their habitats by educating children, teachers, natural resource managers, and the general public. Because birds do not recognize political boundaries, and may even spend most of their lives outside of the United States, RMBO works to bring a unified approach to conservation among states and countries, and many of our projects focus on issues associated with winter grounds, especially those in Mexico. At the core of our conservation work is bird population monitoring. Only through long-term monitoring can we identify which species are in need of help, and evaluate our success at protecting or recovering them. About this manual: This third edition of this manual (formerly entitled Sharing Your Land With Shortgrass Prairie Birds) is about how to help birds and other wildlife make a living from the land while you do the same. -

Effects of Management Practices on Grassland Birds: Bobolink
EFFECTS OF MANAGEMENT PRACTICES ON GRASSLAND BIRDS: BOBOLINK Grasslands Ecosystem Initiative Northern Prairie Wildlife Research Center U.S. Geological Survey Jamestown, North Dakota 58401 This report is one in a series of literature syntheses on North American grassland birds. The need for these reports was identified by the Prairie Pothole Joint Venture (PPJV), a part of the North American Waterfowl Management Plan. The PPJV recently adopted a new goal, to stabilize or increase populations of declining grassland- and wetland-associated wildlife species in the Prairie Pothole Region. To further that objective, it is essential to understand the habitat needs of birds other than waterfowl, and how management practices affect their habitats. The focus of these reports is on management of breeding habitat, particularly in the northern Great Plains. Suggested citation: Dechant, J. A., M. L. Sondreal, D. H. Johnson, L. D. Igl, C. M. Goldade, A. L. Zimmerman, and B. R. Euliss. 1999 (revised 2001). Effects of management practices on grassland birds: Bobolink. Northern Prairie Wildlife Research Center, Jamestown, ND. 24 pages. Species for which syntheses are available or are in preparation: American Bittern Grasshopper Sparrow Mountain Plover Baird’s Sparrow Marbled Godwit Henslow’s Sparrow Long-billed Curlew Le Conte’s Sparrow Willet Nelson’s Sharp-tailed Sparrow Wilson’s Phalarope Vesper Sparrow Upland Sandpiper Savannah Sparrow Greater Prairie-Chicken Lark Sparrow Lesser Prairie-Chicken Field Sparrow Northern Harrier Clay-colored Sparrow Swainson’s Hawk Chestnut-collared Longspur Ferruginous Hawk McCown’s Longspur Short-eared Owl Dickcissel Burrowing Owl Lark Bunting Horned Lark Bobolink Sedge Wren Eastern Meadowlark Loggerhead Shrike Western Meadowlark Sprague’s Pipit Brown-headed Cowbird EFFECTS OF MANAGEMENT PRACTICES ON GRASSLAND BIRDS: BOBOLINK Jill A. -

Bio 209 Course Title: Chordates
BIO 209 CHORDATES NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 209 COURSE TITLE: CHORDATES 136 BIO 209 MODULE 4 MAIN COURSE CONTENTS PAGE MODULE 1 INTRODUCTION TO CHORDATES…. 1 Unit 1 General Characteristics of Chordates………… 1 Unit 2 Classification of Chordates…………………... 6 Unit 3 Hemichordata………………………………… 12 Unit 4 Urochordata………………………………….. 18 Unit 5 Cephalochordata……………………………... 26 MODULE 2 VERTEBRATE CHORDATES (I)……... 31 Unit 1 Vertebrata…………………………………….. 31 Unit 2 Gnathostomata……………………………….. 39 Unit 3 Amphibia…………………………………….. 45 Unit 4 Reptilia……………………………………….. 53 Unit 5 Aves (I)………………………………………. 66 Unit 6 Aves (II)……………………………………… 76 MODULE 3 VERTEBRATE CHORDATES (II)……. 90 Unit 1 Mammalia……………………………………. 90 Unit 2 Eutherians: Proboscidea, Sirenia, Carnivora… 100 Unit 3 Eutherians: Edentata, Artiodactyla, Cetacea… 108 Unit 4 Eutherians: Perissodactyla, Chiroptera, Insectivora…………………………………… 116 Unit 5 Eutherians: Rodentia, Lagomorpha, Primata… 124 MODULE 4 EVOLUTION, ADAPTIVE RADIATION AND ZOOGEOGRAPHY………………. 136 Unit 1 Evolution of Chordates……………………… 136 Unit 2 Adaptive Radiation of Chordates……………. 144 Unit 3 Zoogeography of the Nearctic and Neotropical Regions………………………………………. 149 Unit 4 Zoogeography of the Palaearctic and Afrotropical Regions………………………………………. 155 Unit 5 Zoogeography of the Oriental and Australasian Regions………………………………………. 160 137 BIO 209 CHORDATES COURSE GUIDE BIO 209 CHORDATES Course Team Prof. Ishaya H. Nock (Course Developer/Writer) - ABU, Zaria Prof. T. O. L. Aken’Ova (Course -

Inbreeding in Darwin's Medium Ground Finches (Geospiza Fortis)
Evohaion, 43(6),1989, pp. 1273-1284 INBREEDING IN DARWIN'S MEDIUM GROUND FINCHES (GEOSPIZA FORTIS) H, LISLE GIBBS'" Museum ofZoology and Department ofBiology, The University ofMichigan, Ann Arbor, MI48109-1079 AND PETER R. GRANT Department ofBiology, Princeton University, Princeton, NJ 08544-1008 Abstract. - We studied the frequency and causes of inbreeding and its effect on reproductive success in a population of Darwin's Medium Ground Finches iGeospiza fortis) on Isla Daphne Major, Galapagos, during four breeding seasons (1981, 1983, 1984, and 1987). Pedigree analysis showed that levels of inbreeding were low but comparable with those observed in other passerine birds. For pairs with at least half of their grandparents known, approximately 20% of all pairings were between detectably related birds. The frequency ofpairings between closely related birds (coefficient of kinship [</>] ~ 0.250) among all pairs was 0.6%. We detected no effect of inbreeding on repro ductive success, although sample sizes were small. The observed reproductive output of related pairs was not significantly different from the output ofunrelated pairs, and there was no correlation between a pair's kinship coefficient and an estimate of the potential magnitude of inbreeding depression. Comparisons with a study ofGreat Tits (Parus major) by van Noordwijk and Scharloo (1981) suggest that, even if present, the fitness costs of inbreeding in this population of G. fortis would be low. Observed levels of inbreeding in each breeding episode were accurately predicted by simulations of random mating in which relatedness had no influence on pairing between in dividuals. This result suggests that levels ofinbreeding in this population are determined more by demographic factors than by behavioral avoidance of mating with kin. -

Lark Bunting
This file was created by scanning the printed publication. Errors identified by the software have been corrected; however, some errors may remain. BIOLOGICAL REPORT 82(10.137) MAY 1987 HABITAT SUITABILITY INDEX MODELS: LARK BUNTING , . ', .'~ Fish and Wildlife Service u.s. Department of the Interior MODEL EVALUATION FORM Habitat models are designed for a wide variety of planning applica tions where habitat information is an important consideration in the decision process. However, it is impossible to develop a model that performs equally well in all situations. Assistance from users and researchers is an important part of the model improvement process. Each model is published individually to facilitate updating and reprinting as new information becomes available. User feedback on model performance will assist in improving habitat models for future applications. Please complete this form following application or review of the model. Feel free to include additional information that may be of use to either a model developer or model user. We also would appreciate information on model testing, modification, and application, as well as copies of modified models or test results. Please return this form to: Habitat Evaluation Procedures Group U.S. Fish and Wildlife Service 2627 Redwing Road, Creekside One Fort Collins, CO 80526-2899 Thank you for your assi stance. Geographic Species Location Habitat or Cover Type(s) Type of Application: Impact Analysis Management Action Analysis __ Baseline Other ------------------------- Variables Measured -
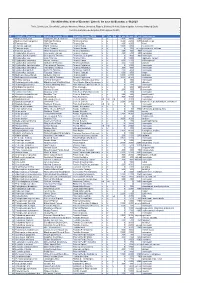
Ecuador Simple List Version 2020 Clements
Checklist of the birds of Ecuador / Lista de las aves del Ecuador, v. 08.2020 Freile, Brinkhuizen, Greenfield, Lysinger, Navarrete, Nilsson, Olmstead, Ridgely, Sánchez-Nivicela, Solano-Ugalde, Athanas, Ahlman & Boyla Comité Ecuatoriano de Registros Ornitológicos (CERO) ID Scientific_Clements_2019 English_Clements_2019 Español Ecuador CERO EC Con Gal Alt_min Alt_max Alt_ext Subespecies 1 Nothocercus julius Tawny-breasted Tinamou Tinamú Pechileonado x x 2300 3400 2100 monotypic 2 Nothocercus bonapartei Highland Tinamou Tinamú Serrano x x 1600 2200 3075 plumbeiceps 3 Tinamus tao Gray Tinamou Tinamú Gris x x 400 1600 kleei 4 Tinamus osgoodi Black Tinamou Tinamú Negro x x 1000 1400 hershkovitzi 5 Tinamus major Great Tinamou Tinamú Grande x x 0 700 1200, 1350 peruvianus, latifrons 6 Tinamus guttatus White-throated Tinamou Tinamú Goliblanco x x 200 400 900 monotypic 7 Crypturellus cinereus Cinereous Tinamou Tinamú Cinéreo x x 200 600 900 monotypic 8 Crypturellus berlepschi Berlepsch's Tinamou Tinamú de Berlepsch x x 0 400 900 monotypic 9 Crypturellus soui Little Tinamou Tinamú Chico x x 0 1200 nigriceps, harterti 10 Crypturellus obsoletus Brown Tinamou Tinamú Pardo x x 500 1100 chirimotanus? 11 Crypturellus undulatus Undulated Tinamou Tinamú Ondulado x x 200 600 yapura 12 Crypturellus transfasciatus Pale-browed Tinamou Tinamú Cejiblanco x x 0 1600 monotypic 13 Crypturellus variegatus Variegated Tinamou Tinamú Abigarrado x x 200 400 monotypic 14 Crypturellus bartletti Bartlett's Tinamou Tinamú de Bartlett x x 200 400 monotypic 15 Crypturellus -

Lark Bunting (Calamospiza Melanocorys): a Technical Conservation Assessment
Lark Bunting (Calamospiza melanocorys): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project March 30, 2006 Diane L. H. Neudorf, Ph.D1, Rebecca A. Bodily1, and Thomas G. Shane2 1 Department of Biological Sciences, Box 2116, Sam Houston State University, Huntsville, TX 77341 2 1706 Belmont, Garden City, KS 67846 Peer Review Administered by Society for Conservation Biology Neudorf, D.L.H., R.A. Bodily, and T.G. Shane. (March 30, 2006). Lark Bunting (Calamospiza melanocorys): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http://www.fs.fed.us/r2/projects/scp/assessments/larkbunting.pdf [date of access]. ACKNOWLEDGMENTS The manuscript was greatly improved by the editorial comments of Greg Hayward and an anonymous reviewer. Mallory Brodrick assisted with management of the literature files. David McDonald provided the matrix model. AUTHOR’S BIOGRAPHY Diane L. H. Neudorf is an Associate Professor of Biology at Sam Houston State University and Director of the Texas Bird Sound Library. She received her Ph.D. in Biology from York University in 1996 for her study of female extra-pair mating tactics in hooded warblers. She obtained her B.S. (1988) and M.S. (1991) degrees in Zoology from the University of Manitoba where she studied host defenses against the brown-headed cowbird. Her current research continues to focus on mating systems and the ecology of brood parasitism in forest-nesting passerines. Rebecca A. Bodily is an Instructor in the Biology Department at Pike’s Peak Community College. She received her bachelor’s degree in Biomedical Science from Texas A&M University in 1998. -

S FINCHES: <I>GEOSPIZA CONIROSTRIS</I>
EVOLUTION INTERNATIONAL JOURNAL OF ORGANIC EVOLUTION PUBLISHED BY THE SOCIETY FOR THE STUDY OF EVOLUTION Vol. 36 July, 1982 No.4 Evolution, 36(4), 1982, pp. 637-657 NICHE SHIFTS AND COMPETITION IN DARWIN'S FINCHES: GEOSPIZA CONIROSTRIS AND CONGENERS B. R. GRANT AND P. R. GRANT Division of Biological Sciences, University of Michigan, Ann Arbor, Michigan 48109 Received February 5, 1981. Revised October 20, 1981 The idea that interspecific competition titative ecological data to the hypotheses, is an important process in structuring because quantitative data were not used communities stems largely from David to construct the hypotheses. In fact, Lack Lack's work with Darwin's Finches (Lack, had almost no ecological data (see Abbott 1940, 1945, 1947, 1969). Lack made eco et al., 1977). logical inferences about feeding niches In our initial studies we analyzed eco from analyses of bill sizes and shapes. He logical and morphological data from six listed several instances where the niches species on eight Galapagos islands (Abbott of coexisting species were different, and et al., 1977; Smith et al., 1978). To follow where the niche of an absent species ap this general approach, we selected for more peared to be occupied by one or more detailed study three situations which have species present. These examples he inter been heralded as especially clear illustra preted as evidence of competitive dis tions of competitive effects (e.g., Lack, placement and exclusion. 1969; Williamson, 1972; Arms and Camp, Lack's aim was to offer a coherent the 1979), the small size of Geospizafortis on oretical framework for understanding the Isla Daphne Major in the absence of G. -

Sharp-Tailed Grouse
NORTHERN GREAT PLAINS JOINT VENTURE PRIORITY BIRD SPECIES August 2012 By: Northern Great Plains Joint Venture Technical Committee 1 TABLE OF CONTENTS Introduction...............................................................3 Methods.....................................................................3 Results.......................................................................7 Baird’s Sparrow...........................................11 Black-billed Cuckoo....................................14 Black-billed Magpie.....................................17 Brewer’s Sparrow........................................20 Burrowing Owl............................................23 Chestnut-collared Longspur........................26 Ferruginous Hawk.......................................29 Grasshopper Sparrow..................................32 Greater Sage-grouse....................................35 Lark Bunting...............................................38 Loggerhead Shrike.......................................41 Long-billed Curlew.....................................44 Mallard.........................................................47 Marbled Godwit..........................................50 McCown’s Longspur...................................53 Mountain Plover..........................................56 Northern Pintail...........................................59 Red-headed Woodpecker............................62 Sharp-tailed Grouse.....................................65 Short-eared Owl..........................................68