Microglial Function and Regulation During Development, Homeostasis and Alzheimer’S Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
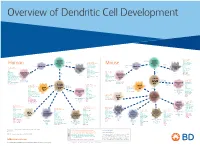
View Dendritic Cell Development Poster
Overview of Dendritic Cell Development Lineage–, CD45+, Common CD117 (c-kit) Common MHCII+, CD11c+ – + CD207 (Langerin) Myeloid CD117 (c-kit) Lineage , CD45 , Myeloid Progenitor MHCII (HLA-DR)+, CD11c+ Progenitor CD324 (E-Cadherin) Human Mouse CD326 (EpCAM) CD207 (Langerin) TGFb1 Cells CD11b, CD115 Cells CD14 Monocyte CD324 (E-Cadherin) Monocyte M-CSF CD11b – + Ly6C Langerhans CD24 Lineage , CD45 , M-CSF CD326 (EpCAM) MHCII (HLA-DR)+, CD11c+ Langerhans CD11blo Zbtb46– Cells CD172a (Sirp-α) CD16 CD1ahi, CD1c CD205 (DEC-205) Cells CSF F4/80 CD64 CD172a (Sirp-α) Lineage–, CD45+, FLT3L TLR3, TLR11 CD1a, CD1c Inflammatory CD369 (Dectin-1/CLEC7A) MHCII+, CD11c+ +/– CSF IL-15 CD8–, CD14– CD11b, CD14 CD371 (CLEC12A) CD64 Monocyte- FLT3L Inflammatory CD370 (Clec9a)– CD172a (Sirp-α) IL-15 CLEC6A CD11b derived lo Monocyte- CD206, CD209 (DC-SIGN) TLR1, TLR2, TLR3 , TLR6 CD209a (DC-SIGN) CD367 (DCIR/CLEC4A) DCs CD14– CD272 (BTLA)lo derived CD369 (Dectin-1/CLEC7A) DCs Common Ly-6C – + CD371 (CLEC12A) CD117 (c-kit) Lineage , CD45 , IL-1β, IL-6, IL-10, TLR1-6, TLR7-8, TLR10 Dendritic + lo CLEC6A – – CD135/FLT3 MHCII , CD11c IL-12, IL-23, TNF CD8a , CD14 IL-1β, IL-6 IL-10, Precursor TLR3lo, TLR4, TLR7, TLR8 CD45R (B220) IL-12, IL-23, TNF Plasmacytoid CD207 (Langerin)– Cells CD317 (BST-2) Common Lineage–, CD45+, FLT3L DCs Lineage–, CD45+, + Ly6C + lo/– CD207 IFN Type I + + Dendritic CD135/FLT3 MHCII (HLA-DR) , CD11c Lineage–, CD45+, IRF7, IRF8, BATF3hi Siglec-H MHCII (HLA-DR) , CD11c hi – + CD123 + + Dermal SpiB, Zbtb46 CD1a, CD64 CD1a Precursor CD117 (c-kit) -

Human Lectins, Their Carbohydrate Affinities and Where to Find Them
biomolecules Review Human Lectins, Their Carbohydrate Affinities and Where to Review HumanFind Them Lectins, Their Carbohydrate Affinities and Where to FindCláudia ThemD. Raposo 1,*, André B. Canelas 2 and M. Teresa Barros 1 1, 2 1 Cláudia D. Raposo * , Andr1 é LAQVB. Canelas‐Requimte,and Department M. Teresa of Chemistry, Barros NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829‐516 Caparica, Portugal; [email protected] 12 GlanbiaLAQV-Requimte,‐AgriChemWhey, Department Lisheen of Chemistry, Mine, Killoran, NOVA Moyne, School E41 of ScienceR622 Co. and Tipperary, Technology, Ireland; canelas‐ [email protected] NOVA de Lisboa, 2829-516 Caparica, Portugal; [email protected] 2* Correspondence:Glanbia-AgriChemWhey, [email protected]; Lisheen Mine, Tel.: Killoran, +351‐212948550 Moyne, E41 R622 Tipperary, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +351-212948550 Abstract: Lectins are a class of proteins responsible for several biological roles such as cell‐cell in‐ Abstract:teractions,Lectins signaling are pathways, a class of and proteins several responsible innate immune for several responses biological against roles pathogens. such as Since cell-cell lec‐ interactions,tins are able signalingto bind to pathways, carbohydrates, and several they can innate be a immuneviable target responses for targeted against drug pathogens. delivery Since sys‐ lectinstems. In are fact, able several to bind lectins to carbohydrates, were approved they by canFood be and a viable Drug targetAdministration for targeted for drugthat purpose. delivery systems.Information In fact, about several specific lectins carbohydrate were approved recognition by Food by andlectin Drug receptors Administration was gathered for that herein, purpose. plus Informationthe specific organs about specific where those carbohydrate lectins can recognition be found by within lectin the receptors human was body. -

Immunology Focus Summer | 2006 Immunology FOCUS
R&D Systems Immunology Focus summer | 2006 Immunology FOCUS Inside page 2 Signal Transduction: Kinase & Phosphatase Reagents page 3 Lectin Family page 4 Regulatory T Cells page 5 Natural Killer Cells page 6 Innate Immunity & Dendritic Cells page 7 Co-Stimulation/-Inhibition The B7 Family & Associated Molecules page 8 Proteome Profiler™ Phospho-Immunoreceptor Array ITAM/ITIM-Associated Receptors www.RnDSystems.com Please visit our website @ www.RnDSystems.com for product information and past issues of the Focus Newsletter: Cancer, Neuroscience, Cell Biology, and more. Quality | Selec tion | Pe rformance | Result s Cancer Development Endocrinology Immunology Neuroscience Proteases Stem Cells Signal Transduction: Kinase & Phosphatase Reagents Co-inhibitory PD-L2/PD-1 signaling & SHP-2 phosphatase KINASE & PHOSPHATASE RESEARCH REAGENTS Regulation of MAP kinase (MAPK) signaling Kinases Phosphatases pathways is critical for T cell development, MOLECULE ANTIBODIES ELISAs/ASSAYS MOLECULE ANTIBODIES ELISAs/ASSAYS activation, differentiation, and death. MAPKs Akt Family H M R H M R Alkaline Phosphatase* H M R are activated by the dual phosphorylation of threonine and tyrosine residues resulting in AMPK H M R Calcineurin A, B H M R subsequent transcription factor activation. ATM H M R H CD45 H M H M The MAPK signaling pathway in T cells can be CaM Kinase II Ms CDC25A, B* H M R triggered by cytokines, growth factors, and CDC2 H M R DARPP-32 M R ligands for transmembrane receptors. Chk1, 2 H M R H M R DEP-1/CD148* H M R H Ligation of the T cell receptor (TCR)/CD3 ERK1, 2 H M R H M R LAR H M R complex results in rapid activation of PI 3- kinase, which leads to Akt and MAPK ERK3 H Lyp H activation. -

Type I Interferon Response Drives Neuroinflammation and Synapse Loss in Alzheimer Disease
RESEARCH ARTICLE The Journal of Clinical Investigation Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease Ethan R. Roy,1,2 Baiping Wang,1 Ying-wooi Wan,3 Gabriel Chiu,1 Allysa Cole,1 Zhuoran Yin,4 Nicholas E. Propson,1,5 Yin Xu,1 Joanna L. Jankowsky,6 Zhandong Liu,7 Virginia M.-Y. Lee,8 John Q. Trojanowski,8 Stephen D. Ginsberg,9,10 Oleg Butovsky,4,11 Hui Zheng,1,3 and Wei Cao1,3 1Huffington Center on Aging, 2Translational Biology & Molecular Medicine Program, and 3Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, Texas, USA. 4Ann Romney Center for Neurologic Diseases, Department of Neurology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA. 5Molecular and Cellular Biology Program, Department of Molecular and Cellular Biology, 6Department of Neuroscience, and 7Department of Pediatrics-Neurology, Baylor College of Medicine, Houston, Texas, USA. 8Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 9Center for Dementia Research, Nathan Kline Institute, Orangeburg, New York, USA. 10Departments of Psychiatry, Neuroscience & Physiology and the NYU Neuroscience Institute, New York University Langone Medical Center, New York, New York, USA. 11Evergrande Center for Immunologic Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA. Type I interferon (IFN) is a key cytokine that curbs viral infection and cell malignancy. Previously, we demonstrated a potent IFN immunogenicity of nucleic acid–containing (NA-containing) amyloid fibrils in the periphery. Here, we investigated whether IFN is associated with β-amyloidosis inside the brain and contributes to neuropathology. -

Influence of Galectin-3 on the Innate Immune Response During
Journal of Fungi Article Influence of Galectin-3 on the Innate Immune Response during Experimental Cryptococcosis Caroline Patini Rezende 1, Patricia Kellen Martins Oliveira Brito 2 , Thiago Aparecido Da Silva 2 , Andre Moreira Pessoni 1 , Leandra Naira Zambelli Ramalho 3 and Fausto Almeida 1,* 1 Department of Biochemistry and Immunology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto 14049-900, SP, Brazil; [email protected] (C.P.R.); [email protected] (A.M.P.) 2 Department of Cellular and Molecular Biology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto 14049-900, SP, Brazil; [email protected] (P.K.M.O.B.); [email protected] (T.A.D.S.) 3 Department of Pathology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto 14049-900, SP, Brazil; [email protected] * Correspondence: [email protected] Abstract: Cryptococcus neoformans, the causative agent of cryptococcosis, is the primary fungal pathogen that affects the immunocompromised individuals. Galectin-3 (Gal-3) is an animal lectin involved in both innate and adaptive immune responses. The present study aimed to evaluate the influence of Gal-3 on the C. neoformans infection. We performed histopathological and gene profile analysis of the innate antifungal immunity markers in the lungs, spleen, and brain of the wild-type (WT) and Gal-3 knockout (KO) mice during cryptococcosis. These findings suggest that Gal-3 absence does not cause significant histopathological alterations in the analyzed tissues. The expression profile of the genes related to innate antifungal immunity showed that the presence of cryptococcosis in Citation: Rezende, C.P.; Brito, the WT and Gal-3 KO animals, compared to their respective controls, promoted the upregulation P.K.M.O.; Da Silva, T.A.; Pessoni, of the pattern recognition receptor (PRR) responsive to mannose/chitin (mrc1) and a gene involved A.M.; Ramalho, L.N.Z.; Almeida, F. -

Immunity Against Fungi
Immunity against fungi Michail S. Lionakis, … , Iliyan D. Iliev, Tobias M. Hohl JCI Insight. 2017;2(11):e93156. https://doi.org/10.1172/jci.insight.93156. Review Pathogenic fungi cause a wide range of syndromes in immune-competent and immune-compromised individuals, with life-threatening disease primarily seen in humans with HIV/AIDS and in patients receiving immunosuppressive therapies for cancer, autoimmunity, and end-organ failure. The discovery that specific primary immune deficiencies manifest with fungal infections and the development of animal models of mucosal and invasive mycoses have facilitated insight into fungus-specific recognition, signaling, effector pathways, and adaptive immune responses. Progress in deciphering the molecular and cellular basis of immunity against fungi is guiding preclinical studies into vaccine and immune reconstitution strategies for vulnerable patient groups. Furthermore, recent work has begun to address the role of endogenous fungal communities in human health and disease. In this review, we summarize a contemporary understanding of protective immunity against fungi. Find the latest version: https://jci.me/93156/pdf REVIEW Immunity against fungi Michail S. Lionakis,1 Iliyan D. Iliev,2 and Tobias M. Hohl3 1Fungal Pathogenesis Unit, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, Maryland, USA. 2Jill Roberts Institute for Research in IBD, Department of Medicine, Weill Cornell Medical College, New York, New York, USA. 3Infectious Disease Service, Department of Medicine, and Immunology Program, Memorial Sloan Kettering Cancer Center, New York, New York, USA. Pathogenic fungi cause a wide range of syndromes in immune-competent and immune- compromised individuals, with life-threatening disease primarily seen in humans with HIV/AIDS and in patients receiving immunosuppressive therapies for cancer, autoimmunity, and end-organ failure. -

Growth Faltering Regardless of Chronic Diarrhea Is Associated with Mucosal Immune Dysfunction and Microbial Dysbiosis in the Gut Lumen
www.nature.com/mi ARTICLE OPEN Growth faltering regardless of chronic diarrhea is associated with mucosal immune dysfunction and microbial dysbiosis in the gut lumen 1 2 3 3 4 5 4 Nicholas S. Rhoades , Sara M. Hendrickson✉ , Kamm Prongay , Andrew Haertel , Leanne Gill , Robert A. Edwards , Laura Garzel , Mark K. Slifka2 and Ilhem Messaoudi1 © The Author(s) 2021 Despite the impact of childhood diarrhea on morbidity and mortality, our understanding of its sequelae has been significantly hampered by the lack of studies that examine samples across the entire intestinal tract. Infant rhesus macaques are naturally susceptible to human enteric pathogens and recapitulate the hallmarks of diarrheal disease such as intestinal inflammation and growth faltering. Here, we examined intestinal biopsies, lamina propria leukocytes, luminal contents, and fecal samples from healthy infants and those experiencing growth faltering with distant acute or chronic active diarrhea. We show that growth faltering in the presence or absence of active diarrhea is associated with a heightened systemic and mucosal pro-inflammatory state centered in the colon. Moreover, polyclonal stimulation of colonic lamina propria leukocytes resulted in a dampened cytokine response, indicative of immune exhaustion. We also detected a functional and taxonomic shift in the luminal microbiome across multiple gut sites including the migration of Streptococcus and Prevotella species between the small and large intestine, suggesting a decompartmentalization of gut microbial communities. Our studies provide valuable insight into the outcomes of diarrheal diseases and growth faltering not attainable in humans and lays the groundwork to test interventions in a controlled and reproducible setting. Mucosal Immunology (2021) 14:1113–1126; https://doi.org/10.1038/s41385-021-00418-2 INTRODUCTION especially in the genus Campylobacter, that are missed by clinical Despite significant improvements in healthcare delivery and tests but highly abundant in developing countries24–26. -

The Innate Immune Receptor Dectin-2 Mediates the Phagocytosis of Cancer Cells by Kupffer Cells for the Suppression of Liver Metastasis
The innate immune receptor Dectin-2 mediates the phagocytosis of cancer cells by Kupffer cells for the suppression of liver metastasis Yoshitaka Kimuraa, Asuka Inouea,b, Sho Hangaia,c, Shinobu Saijod, Hideo Negishia, Junko Nishioa, Sho Yamasakie, Yoichiro Iwakuraf, Hideyuki Yanaia,c, and Tadatsugu Taniguchia,c,1 aDepartment of Molecular Immunology, Institute of Industrial Science, The University of Tokyo, Tokyo 153-8505, Japan; bJapan Research and Open Innovation, Sanofi K.K., Tokyo 163-1488, Japan; cMax Planck–The University of Tokyo Center for Integrative Inflammology, Tokyo 153-8505, Japan; dDepartment of Molecular Immunology, Medical Mycology Research Center, Chiba University, Chiba 260-8673, Japan; eDivision of Molecular Immunology, Medical Institute of Bioregulation, Kyushu University, Fukuoka 812-8582, Japan; and fCenter for Animal Disease Models, Research Institute for Biomedical Sciences, Tokyo University of Science, Chiba 278-0022, Japan Contributed by Tadatsugu Taniguchi, October 30, 2016 (sent for review October 23, 2016; reviewed by Ruslan Medzhitov and Nobuyuki Tanaka) Tumor metastasis is the cause of most cancer deaths. Although also has been reported that Kupffer cells can directly kill cancer metastases can form in multiple end organs, the liver is recognized cells through the secretion of cytotoxic molecules, such as tumor as a highly permissive organ. Nevertheless, there is evidence for necrosis factor (TNF)-α and reactive oxygen species, and Kupffer immune cell-mediated mechanisms that function to suppress liver cells enhance antitumor responses mediated by other immune metastasis by certain tumors, although the underlying mechanisms cells, such as NK cells (9). On the other hand, several reports have forthesuppressionofmetastasisremain elusive. Here, we show that argued that Kupffer cells also have a protumorigenic effect through Dectin-2, a C-type lectin receptor (CLR) family of innate receptors, is the production of inflammatory cytokines and chemokines, which critical for the suppression of liver metastasis of cancer cells. -

SYK Antibody (C-Term) Purified Rabbit Polyclonal Antibody (Pab) Catalog # Ap7720b
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 SYK Antibody (C-term) Purified Rabbit Polyclonal Antibody (Pab) Catalog # AP7720b Specification SYK Antibody (C-term) - Product Information Application WB, IHC-P,E Primary Accession P43405 Other Accession Q64725, Q00655, P48025 Reactivity Human, Mouse Predicted Pig, Rat Host Rabbit Clonality Polyclonal Isotype Rabbit Ig Calculated MW 72066 Antigen Region 387-417 SYK Antibody (C-term) - Additional Information Gene ID 6850 The anti-SYK Pab (Cat. #AP7720b) is used in Western blot to detect SYK in THP-1 cell Other Names lysate (Lane 1) and mouse spleen tissue Tyrosine-protein kinase SYK, Spleen lysate (Lane 2). tyrosine kinase, p72-Syk, SYK Target/Specificity This SYK antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 387-417 amino acids from the C-terminal region of human SYK. Dilution WB~~1:1000 IHC-P~~1:50~100 Format Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is prepared by Saturated Ammonium Sulfate (SAS) precipitation followed by dialysis against PBS. Anti-SYK Antibody (K402) at 1:1000 dilution Storage + Raji whole cell lysate Lysates/proteins at Maintain refrigerated at 2-8°C for up to 2 20 µg per lane. Secondary Goat Anti-Rabbit weeks. For long term storage store at -20°C IgG, (H+L), Peroxidase conjugated at 1/10000 in small aliquots to prevent freeze-thaw dilution. Predicted band size : 72 kDa cycles. Blocking/Dilution buffer: 5% NFDM/TBST. Precautions SYK Antibody (C-term) is for research use Page 1/4 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 only and not for use in diagnostic or therapeutic procedures. -

CLEC7A/Dectin-1 Verringert Die Immunantwort Gegen Sterbende Und Tote Zellen)
CLEC7A/Dectin-1 attenuates the immune response against dying and dead cells (CLEC7A/Dectin-1 verringert die Immunantwort gegen sterbende und tote Zellen) Der Naturwissenschaftlichen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg zur Erlangung des Doktorgrades Dr. rer. nat. vorgelegt von Connie Hesse aus Eberswalde-Finow Als Dissertation genehmigt von der Naturwissenschaftlichen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg Tag der mündlichen Prüfung: 21.12.2010 Vorsitzender der Promotionskommision: Prof. Dr. Rainer Fink Erstberichterstatter: Prof. Dr. Lars Nitschke Zweitberichterstatter: PD Dr. Reinhard Voll Table of Contents Table of Contents Table of Contents ............................................................................................ 1 Abstract ............................................................................................................ 3 Zusammenfassung.......................................................................................... 4 1 Introduction ............................................................................................... 6 1.1 C-type lectins................................................................................................. 8 1.1.1 CLEC4L/DC-SIGN ................................................................................ 11 1.1.2 CLEC7A/Dectin-1.................................................................................. 12 1.1.3 CLEC9A/DNGR1 ................................................................................. -

Analysis of Card9 Signaling in Innate Immunity
! Fakultät für Medizin Institut für Klinische Chemie und Pathobiochemie Analysis of Card9 signaling in innate immunity Susanne Ilona Roth Vollständiger Abdruck der von der Fakultät für Medizin der Technischen Universität München zur Erlangung des akademischen Grades eines Doctor of Philosophy (Ph.D.) genehmigten Dissertation. Vorsitzende: Prof. Dr. Agnes Görlach Betreuer: Prof. Dr. Jürgen Ruland Prüfer der Dissertation: 1. Prof. Dr. Thomas Korn 2. Prof. Dr. Mathias Heikenwälder 3. Prof. Dr. Anne Krug Die Dissertation wurde am 17.06.2017 bei der Fakultät für Medizin der Technischen Universität München eingereicht und durch die Fakultät für Medizin am 21.09.2017 angenommen.! ! Table of content List of Abbreviations 2 1. Introduction 4 1.1 Pattern recognition receptors 4 1.2 Cytosolic DNA recognition receptors 5 1.3 Syk-coupled C-type lectin receptors 7 1.4 Card9 structure, expression and biochemistry 10 1.5 Card9-mediated PRR signaling 10 1.6 CLR-Card9 signaling in anti-fungal host defense 12 1.7 Card9 in inflammatory diseases 12 2. Aim of the present study and scientific approach 13 3. Results and discussion 14 3.1 Role of Card9 in cytosolic DNA-induced inflammatory responses 14 3.2 Vav proteins control Syk-coupled C-type lectin receptor triggered inflammatory responses via Card9-Bcl10-Malt1 signalosomes 18 3.3 Dectin-1-Syk-Card9-IRF5 signaling regulates Interferon-! responses in anti- fungal immunity 20 4. Summary of each publication and individual contribution of the candidate 21 4.1 Rad50-Card9 interactions link cytosolic DNA sensing to IL-1! production 21 4.2 Vav Proteins Are Key Regulators of Card9 Signaling for Innate Antifungal Immunity 22 4.3 Interferon-! Production via Dectin-1-Syk-IRF5 Signaling in Dendritic Cells Is Crucial for Immunity to C. -

B-Glucans in Food Modify Colonic Microflora by Inducing Antimicrobial Protein, Calprotectin, in a Dectin-1-Induced-IL-17F-Dependent Manner
ARTICLES b-Glucans in food modify colonic microflora by inducing antimicrobial protein, calprotectin, in a Dectin-1-induced-IL-17F-dependent manner T Kamiya1,2,3, C Tang2,3,4, M Kadoki2,3, K Oshima5, M Hattori5, S Saijo3,6, Y Adachi7, N Ohno7 and Y Iwakura1,2,3,4,5,6 Dectin-1 (gene symbol: Clec7a) is a receptor for b-glucans that play an important role for the host defense against fungi. Recently, we showed that Clec7a À / À mice are resistant against dextran sodium sulfate (DSS)-induced colitis because of regulatory T-cell population expansion in the colon. The regulatory T-cell expansion is caused by expansion of commensal Lactobacillus murinus whose growth is suppressed by an antimicrobial protein, calprotectin S100A8/A9. In this report, we showed that S100A8 was mainly produced by mouse colonic epithelial cells. S100A8 was not induced directly by Dectin-1 but by Dectin-1-induced cytokines, especially interleukin-17F (IL-17F), that were produced by several types of innate immune cells including CD11c þ /CD11b þ myeloid cells in colonic lamina propria. S100A8/A9 heterodimer preferentially suppressed the growth of L. murinus that was increased in both Clec7a À / À and Il17f À / À mice. Furthermore, similar expansion of L. murinus and DSS-colitis resistance were observed in mice fed with b-glucan- free food. These observations suggest that food-derived b-glucans control the specific commensal microbiota via the Dectin-1–IL-17F–calprotectin axis to maintain the intestinal homeostasis. INTRODUCTION pathogens and intestinal immune homeostasis.9,10 C-type Our intestinal tract is colonized by many types of microbiota.