B-Glucans in Food Modify Colonic Microflora by Inducing Antimicrobial Protein, Calprotectin, in a Dectin-1-Induced-IL-17F-Dependent Manner
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Network Medicine Approach for Analysis of Alzheimer's Disease Gene Expression Data
International Journal of Molecular Sciences Article Network Medicine Approach for Analysis of Alzheimer’s Disease Gene Expression Data David Cohen y, Alexander Pilozzi y and Xudong Huang * Neurochemistry Laboratory, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA; [email protected] (D.C.); [email protected] (A.P.) * Correspondence: [email protected]; Tel./Fax: +1-617-724-9778 These authors contributed equally to this work. y Received: 15 November 2019; Accepted: 30 December 2019; Published: 3 January 2020 Abstract: Alzheimer’s disease (AD) is the most widespread diagnosed cause of dementia in the elderly. It is a progressive neurodegenerative disease that causes memory loss as well as other detrimental symptoms that are ultimately fatal. Due to the urgent nature of this disease, and the current lack of success in treatment and prevention, it is vital that different methods and approaches are applied to its study in order to better understand its underlying mechanisms. To this end, we have conducted network-based gene co-expression analysis on data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. By processing and filtering gene expression data taken from the blood samples of subjects with varying disease states and constructing networks based on that data to evaluate gene relationships, we have been able to learn about gene expression correlated with the disease, and we have identified several areas of potential research interest. Keywords: Alzheimer’s disease; network medicine; gene expression; neurodegeneration; neuroinflammation 1. Introduction Alzheimer’s disease (AD) is the most widespread diagnosed cause of dementia in the elderly [1]. -

Systems and Chemical Biology Approaches to Study Cell Function and Response to Toxins
Dissertation submitted to the Combined Faculties for the Natural Sciences and for Mathematics of the Ruperto-Carola University of Heidelberg, Germany for the degree of Doctor of Natural Sciences Presented by MSc. Yingying Jiang born in Shandong, China Oral-examination: Systems and chemical biology approaches to study cell function and response to toxins Referees: Prof. Dr. Rob Russell Prof. Dr. Stefan Wölfl CONTRIBUTIONS The chapter III of this thesis was submitted for publishing under the title “Drug mechanism predominates over toxicity mechanisms in drug induced gene expression” by Yingying Jiang, Tobias C. Fuchs, Kristina Erdeljan, Bojana Lazerevic, Philip Hewitt, Gordana Apic & Robert B. Russell. For chapter III, text phrases, selected tables, figures are based on this submitted manuscript that has been originally written by myself. i ABSTRACT Toxicity is one of the main causes of failure during drug discovery, and of withdrawal once drugs reached the market. Prediction of potential toxicities in the early stage of drug development has thus become of great interest to reduce such costly failures. Since toxicity results from chemical perturbation of biological systems, we combined biological and chemical strategies to help understand and ultimately predict drug toxicities. First, we proposed a systematic strategy to predict and understand the mechanistic interpretation of drug toxicities based on chemical fragments. Fragments frequently found in chemicals with certain toxicities were defined as structural alerts for use in prediction. Some of the predictions were supported with mechanistic interpretation by integrating fragment- chemical, chemical-protein, protein-protein interactions and gene expression data. Next, we systematically deciphered the mechanisms of drug actions and toxicities by analyzing the associations of drugs’ chemical features, biological features and their gene expression profiles from the TG-GATEs database. -

Microglial Function and Regulation During Development, Homeostasis and Alzheimer’S Disease
cells Review Microglial Function and Regulation during Development, Homeostasis and Alzheimer’s Disease Brad T. Casali and Erin G. Reed-Geaghan * Department of Pharmaceutical Sciences, Northeast Ohio Medical University, Rootstown, OH 44272, USA; [email protected] * Correspondence: [email protected] Abstract: Microglia are the resident immune cells of the brain, deriving from yolk sac progenitors that populate the brain parenchyma during development. During development and homeostasis, microglia play critical roles in synaptogenesis and synaptic plasticity, in addition to their primary role as immune sentinels. In aging and neurodegenerative diseases generally, and Alzheimer’s disease (AD) specifically, microglial function is altered in ways that significantly diverge from their homeostatic state, inducing a more detrimental inflammatory environment. In this review, we discuss the receptors, signaling, regulation and gene expression patterns of microglia that mediate their phenotype and function contributing to the inflammatory milieu of the AD brain, as well as strategies that target microglia to ameliorate the onset, progression and symptoms of AD. Keywords: microglia; inflammation; Alzheimer’s disease; neurodegenerative diseases; TREM2; neu- roinflammation Citation: Casali, B.T.; Reed-Geaghan, 1. Introduction E.G. Microglial Function and Microglia are the resident phagocytes of the central nervous system (CNS). In addition Regulation during Development, to their immunological role in maintaining CNS homeostasis, microglia play vital -

Enteric Alpha Defensins in Norm and Pathology Nikolai a Lisitsyn1*, Yulia a Bukurova1, Inna G Nikitina1, George S Krasnov1, Yuri Sykulev2 and Sergey F Beresten1
Lisitsyn et al. Annals of Clinical Microbiology and Antimicrobials 2012, 11:1 http://www.ann-clinmicrob.com/content/11/1/1 REVIEW Open Access Enteric alpha defensins in norm and pathology Nikolai A Lisitsyn1*, Yulia A Bukurova1, Inna G Nikitina1, George S Krasnov1, Yuri Sykulev2 and Sergey F Beresten1 Abstract Microbes living in the mammalian gut exist in constant contact with immunity system that prevents infection and maintains homeostasis. Enteric alpha defensins play an important role in regulation of bacterial colonization of the gut, as well as in activation of pro- and anti-inflammatory responses of the adaptive immune system cells in lamina propria. This review summarizes currently available data on functions of mammalian enteric alpha defensins in the immune defense and changes in their secretion in intestinal inflammatory diseases and cancer. Keywords: Enteric alpha defensins, Paneth cells, innate immunity, IBD, colon cancer Introduction hydrophobic structure with a positively charged hydro- Defensins are short, cysteine-rich, cationic peptides philic part) is essential for the insertion into the micro- found in vertebrates, invertebrates and plants, which bial membrane and the formation of a pore leading to play an important role in innate immunity against bac- membrane permeabilization and lysis of the microbe teria, fungi, protozoa, and viruses [1]. Mammalian [10]. Initial recognition of numerous microbial targets is defensins are predominantly expressed in epithelial cells a consequence of electrostatic interactions between the of skin, respiratory airways, gastrointestinal and geni- defensins arginine residues and the negatively charged tourinary tracts, which form physical barriers to external phospholipids of the microbial cytoplasmic membrane infectious agents [2,3], and also in leukocytes (mostly [2,5]. -
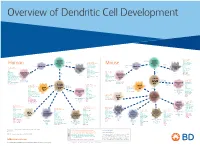
View Dendritic Cell Development Poster
Overview of Dendritic Cell Development Lineage–, CD45+, Common CD117 (c-kit) Common MHCII+, CD11c+ – + CD207 (Langerin) Myeloid CD117 (c-kit) Lineage , CD45 , Myeloid Progenitor MHCII (HLA-DR)+, CD11c+ Progenitor CD324 (E-Cadherin) Human Mouse CD326 (EpCAM) CD207 (Langerin) TGFb1 Cells CD11b, CD115 Cells CD14 Monocyte CD324 (E-Cadherin) Monocyte M-CSF CD11b – + Ly6C Langerhans CD24 Lineage , CD45 , M-CSF CD326 (EpCAM) MHCII (HLA-DR)+, CD11c+ Langerhans CD11blo Zbtb46– Cells CD172a (Sirp-α) CD16 CD1ahi, CD1c CD205 (DEC-205) Cells CSF F4/80 CD64 CD172a (Sirp-α) Lineage–, CD45+, FLT3L TLR3, TLR11 CD1a, CD1c Inflammatory CD369 (Dectin-1/CLEC7A) MHCII+, CD11c+ +/– CSF IL-15 CD8–, CD14– CD11b, CD14 CD371 (CLEC12A) CD64 Monocyte- FLT3L Inflammatory CD370 (Clec9a)– CD172a (Sirp-α) IL-15 CLEC6A CD11b derived lo Monocyte- CD206, CD209 (DC-SIGN) TLR1, TLR2, TLR3 , TLR6 CD209a (DC-SIGN) CD367 (DCIR/CLEC4A) DCs CD14– CD272 (BTLA)lo derived CD369 (Dectin-1/CLEC7A) DCs Common Ly-6C – + CD371 (CLEC12A) CD117 (c-kit) Lineage , CD45 , IL-1β, IL-6, IL-10, TLR1-6, TLR7-8, TLR10 Dendritic + lo CLEC6A – – CD135/FLT3 MHCII , CD11c IL-12, IL-23, TNF CD8a , CD14 IL-1β, IL-6 IL-10, Precursor TLR3lo, TLR4, TLR7, TLR8 CD45R (B220) IL-12, IL-23, TNF Plasmacytoid CD207 (Langerin)– Cells CD317 (BST-2) Common Lineage–, CD45+, FLT3L DCs Lineage–, CD45+, + Ly6C + lo/– CD207 IFN Type I + + Dendritic CD135/FLT3 MHCII (HLA-DR) , CD11c Lineage–, CD45+, IRF7, IRF8, BATF3hi Siglec-H MHCII (HLA-DR) , CD11c hi – + CD123 + + Dermal SpiB, Zbtb46 CD1a, CD64 CD1a Precursor CD117 (c-kit) -

Duplications and Copy Number Variants of 8P23.1 Are Cytogenetically Indistinguishable but Distinct at the Molecular Level
European Journal of Human Genetics (2005) 13, 1131–1136 & 2005 Nature Publishing Group All rights reserved 1018-4813/05 $30.00 www.nature.com/ejhg ARTICLE Duplications and copy number variants of 8p23.1 are cytogenetically indistinguishable but distinct at the molecular level John CK Barber*,1,2,3, Viv Maloney2, Edward J Hollox4, Annegret Stuke-Sontheimer5, Gabi du Bois6, Eva Daumiller6, Ute Klein-Vogler7, Andreas Dufke7, John AL Armour4 and Thomas Liehr8 1Wessex Regional Genetics Laboratory, Salisbury Hospital NHS Trust, Salisbury, Wiltshire, UK; 2National Genetics Reference Laboratory (Wessex), Salisbury Hospital NHS Trust, Salisbury, Wiltshire, UK; 3Human Genetics Division, Southampton University School of Medicine, Southampton General Hospital, Southampton, UK; 4Institute of Genetics, University of Nottingham, Queen’s Medical Centre, Nottingham, UK; 5Genetics Clinic, Wernigerode, Germany; 6Institute for Chromosome Diagnostics and Genetic Counselling, Boeblingen, Germany; 7Department of Medical Genetics, Eberhard-Karls University, Tuebingen, Germany; 8Institute for Human Genetics and Anthropology, Friedrich-Schiller University, Jena, Germany It has been proposed that duplications of 8p23.1 are either euchromatic variants of the 8p23.1 defensin domain with no phenotypic consequences or true duplications associated with developmental delay and heart defects. Here, we provide evidence for both alternatives in two new families. A duplication of most of band 8p23.1 (circa 5 Mb) was found in a girl of 8 years with pulmonary stenosis and mild language delay. BAC fluorescence in situ hybridisation (FISH) and multiplex amplifiable probe hybridisation (MAPH) showed that the two copies of the duplicated segment were sited, in an alternating fashion, between three copies of a circa 300–450 kb segment from 8p23.1 distal to REPD. -

Human Lectins, Their Carbohydrate Affinities and Where to Find Them
biomolecules Review Human Lectins, Their Carbohydrate Affinities and Where to Review HumanFind Them Lectins, Their Carbohydrate Affinities and Where to FindCláudia ThemD. Raposo 1,*, André B. Canelas 2 and M. Teresa Barros 1 1, 2 1 Cláudia D. Raposo * , Andr1 é LAQVB. Canelas‐Requimte,and Department M. Teresa of Chemistry, Barros NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829‐516 Caparica, Portugal; [email protected] 12 GlanbiaLAQV-Requimte,‐AgriChemWhey, Department Lisheen of Chemistry, Mine, Killoran, NOVA Moyne, School E41 of ScienceR622 Co. and Tipperary, Technology, Ireland; canelas‐ [email protected] NOVA de Lisboa, 2829-516 Caparica, Portugal; [email protected] 2* Correspondence:Glanbia-AgriChemWhey, [email protected]; Lisheen Mine, Tel.: Killoran, +351‐212948550 Moyne, E41 R622 Tipperary, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +351-212948550 Abstract: Lectins are a class of proteins responsible for several biological roles such as cell‐cell in‐ Abstract:teractions,Lectins signaling are pathways, a class of and proteins several responsible innate immune for several responses biological against roles pathogens. such as Since cell-cell lec‐ interactions,tins are able signalingto bind to pathways, carbohydrates, and several they can innate be a immuneviable target responses for targeted against drug pathogens. delivery Since sys‐ lectinstems. In are fact, able several to bind lectins to carbohydrates, were approved they by canFood be and a viable Drug targetAdministration for targeted for drugthat purpose. delivery systems.Information In fact, about several specific lectins carbohydrate were approved recognition by Food by andlectin Drug receptors Administration was gathered for that herein, purpose. plus Informationthe specific organs about specific where those carbohydrate lectins can recognition be found by within lectin the receptors human was body. -

Immunology Focus Summer | 2006 Immunology FOCUS
R&D Systems Immunology Focus summer | 2006 Immunology FOCUS Inside page 2 Signal Transduction: Kinase & Phosphatase Reagents page 3 Lectin Family page 4 Regulatory T Cells page 5 Natural Killer Cells page 6 Innate Immunity & Dendritic Cells page 7 Co-Stimulation/-Inhibition The B7 Family & Associated Molecules page 8 Proteome Profiler™ Phospho-Immunoreceptor Array ITAM/ITIM-Associated Receptors www.RnDSystems.com Please visit our website @ www.RnDSystems.com for product information and past issues of the Focus Newsletter: Cancer, Neuroscience, Cell Biology, and more. Quality | Selec tion | Pe rformance | Result s Cancer Development Endocrinology Immunology Neuroscience Proteases Stem Cells Signal Transduction: Kinase & Phosphatase Reagents Co-inhibitory PD-L2/PD-1 signaling & SHP-2 phosphatase KINASE & PHOSPHATASE RESEARCH REAGENTS Regulation of MAP kinase (MAPK) signaling Kinases Phosphatases pathways is critical for T cell development, MOLECULE ANTIBODIES ELISAs/ASSAYS MOLECULE ANTIBODIES ELISAs/ASSAYS activation, differentiation, and death. MAPKs Akt Family H M R H M R Alkaline Phosphatase* H M R are activated by the dual phosphorylation of threonine and tyrosine residues resulting in AMPK H M R Calcineurin A, B H M R subsequent transcription factor activation. ATM H M R H CD45 H M H M The MAPK signaling pathway in T cells can be CaM Kinase II Ms CDC25A, B* H M R triggered by cytokines, growth factors, and CDC2 H M R DARPP-32 M R ligands for transmembrane receptors. Chk1, 2 H M R H M R DEP-1/CD148* H M R H Ligation of the T cell receptor (TCR)/CD3 ERK1, 2 H M R H M R LAR H M R complex results in rapid activation of PI 3- kinase, which leads to Akt and MAPK ERK3 H Lyp H activation. -

Type I Interferon Response Drives Neuroinflammation and Synapse Loss in Alzheimer Disease
RESEARCH ARTICLE The Journal of Clinical Investigation Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease Ethan R. Roy,1,2 Baiping Wang,1 Ying-wooi Wan,3 Gabriel Chiu,1 Allysa Cole,1 Zhuoran Yin,4 Nicholas E. Propson,1,5 Yin Xu,1 Joanna L. Jankowsky,6 Zhandong Liu,7 Virginia M.-Y. Lee,8 John Q. Trojanowski,8 Stephen D. Ginsberg,9,10 Oleg Butovsky,4,11 Hui Zheng,1,3 and Wei Cao1,3 1Huffington Center on Aging, 2Translational Biology & Molecular Medicine Program, and 3Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, Texas, USA. 4Ann Romney Center for Neurologic Diseases, Department of Neurology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA. 5Molecular and Cellular Biology Program, Department of Molecular and Cellular Biology, 6Department of Neuroscience, and 7Department of Pediatrics-Neurology, Baylor College of Medicine, Houston, Texas, USA. 8Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 9Center for Dementia Research, Nathan Kline Institute, Orangeburg, New York, USA. 10Departments of Psychiatry, Neuroscience & Physiology and the NYU Neuroscience Institute, New York University Langone Medical Center, New York, New York, USA. 11Evergrande Center for Immunologic Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA. Type I interferon (IFN) is a key cytokine that curbs viral infection and cell malignancy. Previously, we demonstrated a potent IFN immunogenicity of nucleic acid–containing (NA-containing) amyloid fibrils in the periphery. Here, we investigated whether IFN is associated with β-amyloidosis inside the brain and contributes to neuropathology. -

Influence of Galectin-3 on the Innate Immune Response During
Journal of Fungi Article Influence of Galectin-3 on the Innate Immune Response during Experimental Cryptococcosis Caroline Patini Rezende 1, Patricia Kellen Martins Oliveira Brito 2 , Thiago Aparecido Da Silva 2 , Andre Moreira Pessoni 1 , Leandra Naira Zambelli Ramalho 3 and Fausto Almeida 1,* 1 Department of Biochemistry and Immunology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto 14049-900, SP, Brazil; [email protected] (C.P.R.); [email protected] (A.M.P.) 2 Department of Cellular and Molecular Biology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto 14049-900, SP, Brazil; [email protected] (P.K.M.O.B.); [email protected] (T.A.D.S.) 3 Department of Pathology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto 14049-900, SP, Brazil; [email protected] * Correspondence: [email protected] Abstract: Cryptococcus neoformans, the causative agent of cryptococcosis, is the primary fungal pathogen that affects the immunocompromised individuals. Galectin-3 (Gal-3) is an animal lectin involved in both innate and adaptive immune responses. The present study aimed to evaluate the influence of Gal-3 on the C. neoformans infection. We performed histopathological and gene profile analysis of the innate antifungal immunity markers in the lungs, spleen, and brain of the wild-type (WT) and Gal-3 knockout (KO) mice during cryptococcosis. These findings suggest that Gal-3 absence does not cause significant histopathological alterations in the analyzed tissues. The expression profile of the genes related to innate antifungal immunity showed that the presence of cryptococcosis in Citation: Rezende, C.P.; Brito, the WT and Gal-3 KO animals, compared to their respective controls, promoted the upregulation P.K.M.O.; Da Silva, T.A.; Pessoni, of the pattern recognition receptor (PRR) responsive to mannose/chitin (mrc1) and a gene involved A.M.; Ramalho, L.N.Z.; Almeida, F. -

Immunity Against Fungi
Immunity against fungi Michail S. Lionakis, … , Iliyan D. Iliev, Tobias M. Hohl JCI Insight. 2017;2(11):e93156. https://doi.org/10.1172/jci.insight.93156. Review Pathogenic fungi cause a wide range of syndromes in immune-competent and immune-compromised individuals, with life-threatening disease primarily seen in humans with HIV/AIDS and in patients receiving immunosuppressive therapies for cancer, autoimmunity, and end-organ failure. The discovery that specific primary immune deficiencies manifest with fungal infections and the development of animal models of mucosal and invasive mycoses have facilitated insight into fungus-specific recognition, signaling, effector pathways, and adaptive immune responses. Progress in deciphering the molecular and cellular basis of immunity against fungi is guiding preclinical studies into vaccine and immune reconstitution strategies for vulnerable patient groups. Furthermore, recent work has begun to address the role of endogenous fungal communities in human health and disease. In this review, we summarize a contemporary understanding of protective immunity against fungi. Find the latest version: https://jci.me/93156/pdf REVIEW Immunity against fungi Michail S. Lionakis,1 Iliyan D. Iliev,2 and Tobias M. Hohl3 1Fungal Pathogenesis Unit, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, Maryland, USA. 2Jill Roberts Institute for Research in IBD, Department of Medicine, Weill Cornell Medical College, New York, New York, USA. 3Infectious Disease Service, Department of Medicine, and Immunology Program, Memorial Sloan Kettering Cancer Center, New York, New York, USA. Pathogenic fungi cause a wide range of syndromes in immune-competent and immune- compromised individuals, with life-threatening disease primarily seen in humans with HIV/AIDS and in patients receiving immunosuppressive therapies for cancer, autoimmunity, and end-organ failure. -

Systemic Upregulation of Neutrophil A-Defensins and Serine Proteases In
Asthma Systemic upregulation of neutrophil a-defensins and Thorax: first published as 10.1136/thx.2010.157719 on 23 July 2011. Downloaded from serine proteases in neutrophilic asthma Katherine J Baines,1,2 Jodie L Simpson,1,2 Lisa G Wood,1,2 Rodney J Scott,3 Peter G Gibson1,2 1Priority Research Centre for ABSTRACT Asthma and Respiratory Background The well-characterised airway inflammatory Key messages Diseases, The University of phenotypes of asthma include eosinophilic, neutrophilic, Newcastle, Callaghan, Australia 2Department of Respiratory and mixed eosinophilic/neutrophilic and paucigranulocytic What is the key question? Sleep Medicine, Hunter Medical asthma, identified based on the proportion of sputum < Are systemic gene expression profiles different Research Institute, John Hunter granulocytes. Systemic inflammation is now recognised between inflammatory phenotypes of asthma? Hospital, New Lambton, as an important part of some airway diseases, but the Australia 3 involvement of systemic inflammation in the What is the bottom line? Priority Research Centre of < Information Based Medicine, pathogenesis of airway inflammatory phenotypes of The neutrophilic phenotype of asthma is Hunter Medical Research asthma remains unknown. associated with increased systemic gene Institute, The University of Methods Induced sputum samples and peripheral blood expression of neutrophil a-defensins and prote- Newcastle, Callaghan, Australia were collected from participants with asthma (n¼36). ases elastase and cathepsin G. Airway inflammatory cell counts were performed from Correspondence to Why read on? Dr Katherine J Baines, Level 3, induced sputum and inflammatory phenotype assigned < HMRI, John Hunter Hospital, based on the airway eosinophil and neutrophil cut-offs of This study explores the relationship between Locked Bag 1, Hunter Region 3% and 61%, respectively.